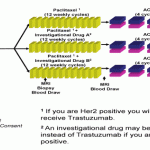
adaptive clinical trials
The approval of new drugs and medical devices is a process fraught with scientific, political, and ethical landmines. Inherent in any such process is an unavoidable conflict between rigorous science and safety on the one side, which tend to slow the process down by requiring large randomized clinical trials that can take years, versus forces that demand faster approval. For example, patients suffering from deadly diseases demand faster approval of drugs that might give them the hope of surviving their disease, or at least of surviving considerably longer. This is a powerful force for reform,…
As I write this, I'm winging my way home from TAM, crammed uncomfortably—very uncomfortably—in a window seat in steerage—I mean, coach).
I had thought of simply recounting the adventures of the contingent of skeptics with whom I'm associated who did make it out to TAM to give talks at workshops and the main stage and to be on panels, but that seemed too easy. Also Orac is just too damned egotistical, and, besides, it's a four hour flight. Even so, I would be remiss if, before delving into the topic of today's post, I didn't praise Steve Novella, Harriet Hall, and Mark Crislip, for their…