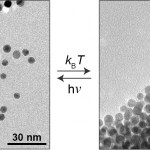
One day in the future, we may be treating our ailments with microbiotic combinations designed specifically to correct imbalances in our personal microbiomes. We’ll bring our prescriptions on rewritable paper and pay using shimmery optical chips embedded in our cell phone cases or maybe our jewelry. Or we’ll be waiting in our doctor’s office for a simple test of our microbiogenome to see if a light-based nanoparticle delivery treatment is working, while watching iridescent optical displays that change as we move...
These future scenarios (and many more) are all imaginary, but…
organic chemistry
Two completely unrelated papers have got us thinking about chemical bonds. When we refer to chemical bonds, we generally mean covalent bonds: Atoms become "wedded," sharing electrons, and breaking them apart takes energy. By comparison, other types of bonds are weak attractions - mere flirtations, or mild sparks between hydrogen and oxygen in passing water molecules.
So why would a researcher in organic chemistry - a field based on carbon, the king of covalent bonds - be investigating the properties of non-covalent, hydrophobic bonds? The answer, of course, is that they can be used to create…