thermochemistry
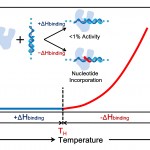
Our lab has a paper called: "Enthalpic Switch-Points and Temperature Dependencies of DNA binding and Nucleotide Incorporation by Pol I DNA Polymerases" that was just published in BBA (Biochimica et Biophysica Acta): Proteins and Proteomics. The study follows up on an observation and prediction we had made some years ago in a different paper.
The study deals with quite a lot of rather detailed thermodynamics of DNA binding (free energy, enthalpy, entropy, heat capacity…) and looks for correlations between such thermodynamic measurements of binding and the functional behavior of a couple…
Yanling Yang, who just graduated with a Ph.D. from my lab, has a paper in the just published November issue of Biophysical Chemistry. The entire issue of the journal celebrates the 25th Anniversary of a conference called "The Gibbs Conference on Biothermodynamics", and each of the papers is from the laboratory of one of the organizers of one of the previous 25 annual meetings (I co-organized #24). Despite the restricted invitation list, however, all the papers were peer reviewed (some quite viciously according to reports) and some required several months of revisions to qualify for the…
Okay, after a long, long gap (on the blogosphere timescale) and/or almost zero elapsed time (by scientific literature standards), we're going to attempt to wrap up this mini-series on heat capacity effects in biology. Parts 1 and 2 are here and here, respectively.
So: How do you know if your reaction has a heat capacity change? Actually, it's easy: collect data as a function of temperature and make a van't Hoff plot (ln 1/Keq versus ln 1/temperture) or a Gibbs-Helmholtz plot (ÎG versus temperature) - if either plot is not-linear your reaction has a ÎCp.
This figure shows Gibbs-Helmholtz…
The laws of thermodynamics are empirical laws - they were not derived from some first principles of the universe: they were derived by doing thousands and thousands of experiments, and then coming up with some relationships that could quantitatively explain all those experiments.
In biological thermodynamics, we are at the beginnings of trying to define a similar set of "laws of biothermodynamics" - in this case we want relationships that connect thermodynamic quantities (ÎG, ÎH, ÎS, ÎCp) to functional or structural information about the biomolecules themselves. Nobody has anything that…
Scienceblogs is promoting the writing of "Science 101" general topic posts all through the "back to school" month of September. So, here is the first in a multi-part series on Heat Capacity in Biology:
Heat Capacity in Biology 101: What is it?
Heat capacity is basically a proportionality constant. For any substance, the heat capacity tells you how much the temperature of the substance will change when you add a specific amount of heat.
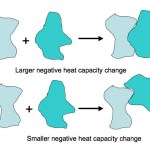
Here is an absolutely beautiful schematic illustration of the difference between a small heat capacity and a large heat capacity (from a website on the…