When I was first learning about the science of the Moon, there were a few basic facts that everyone got right. The Moon has practically no atmosphere, as when sunlight hits the Moon, it very quickly can give individual molecules and atoms enough energy to achieve escape velocity. We thought the same thing was true for any water on the Moon; sunlight would kick the water molecules so hard and so often that the molecules wouldn't remain on the Moon for very long, and therefore it would be completely dry.

A watery Moon? Possible at all? Well, there was one hope. You see, the Earth rotates on an axis tilted at about 23 degrees around the Sun. But the Moon is barely tilted at all, with an axial tilt of less than 2 degrees. Well, that tilt is so tiny that it's possible that the interior of deep craters at the North and South Poles of the Moon might never see sunlight. Check out this crater at a latitude of 89.54 degrees South, and see what I'm talking about:

Those places -- it was thought -- were the best bets for finding water on the Moon. But too much continuous sunlight was thought to kill the hope for water on the vast majority of the Moon. Even when the Apollo missions brought back Moonrocks that contained a little bit of water, it was assumed that the water was contamination from the humid Houston air.
Well, the Indian mission, Chandrayaan-1, completed its mineral analysis of the Moon, and guess what it found? Well, why bother telling you when I can show you. You see, water (and the hydroxyl ion, OH-) absorbs light at very particular wavelengths. If the Moon contained water, we would expect a drop in the reflectance of the Moon's surface, like so:
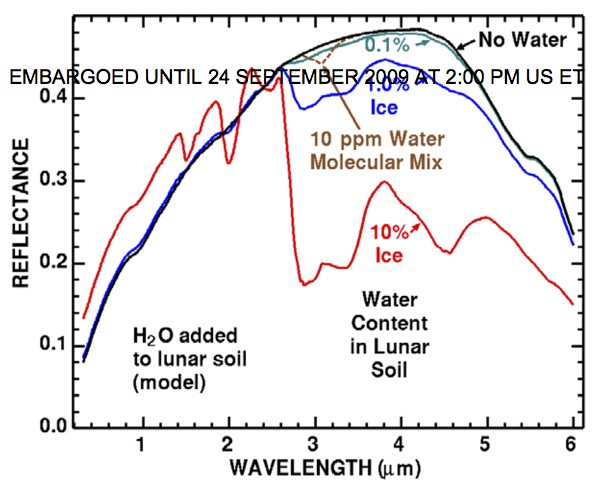
Well, what did Chandrayaan-1 find? There are three papers being released today at 2:00 PM Eastern Time: one by Roger Clark, one by Paul Lucey, and one by Carle Pieters (and the rest of the Chandrayaan team). I owe my copies to the generosity of another scienceblogger, who was one of only a few hundred people to find out this news before the press leaked it. Not only do these teams find the dip in the spectrum characteristic of water:
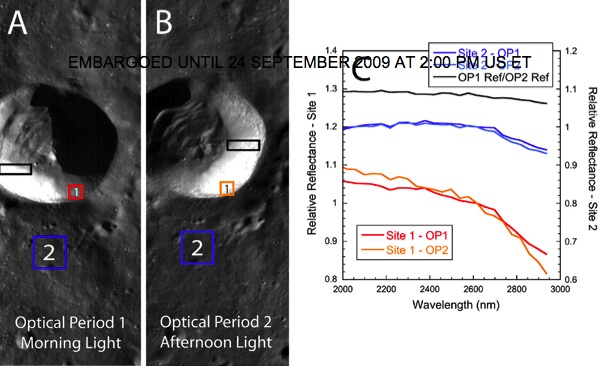
They find it everywhere on the Moon, and just millimeters below the surface! Their estimates from the data show that for every cubic meter of the Moon's soil, there is approximately one liter of water buried (and probably frozen) there.
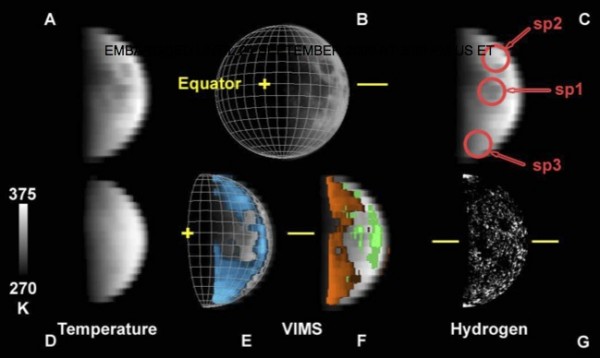
Check out the signals; there's water everywhere, even in areas constantly bathed in sunlight. This could be a huge boon to establishing a Moon base, as with water (or even hydroxyl ions) on the surface, we could possibly generate our own liquid water and breathable O2 gas!
And what an amazing breakthrough it is for the Indian space program to make this find!
This is a great day for lunar science! Perhaps you'll look at our Moon a little differently now; I know that I will.


Very interesting but shouldn't you have respected their embargo for just two more hours?
I ponder if you would want your paper leaked ahead of your very big discovery.
In 1973 Science published an article with evidence that water was produced in dehydrated lunar samples that were exposed to hydrogen. The mechanism involved reaction between hydrogen and the oxygen in breccia. However, they concluded that this would only lead to water vapor in the atmosphere due to the high surface temperature, and they felt that this type of water formation was only significant for planets having reducing atmospheres and enough gravity to hold on to the water vapor. They did not believe that the water would remain on the lunar surface because the temperature was too high during the daytime.
see Cadenhead and Buergel, Science, Vol. 180, 15 June 1973, p.1166
I think its cool that there is water on the moon but what does that mean for us will there ever be a chance to live there ???
Here is what I don't understand.
Why didn't NASA back in the 1970s bombard lunar samples with D+ to see if they could form DOH that would survive the conditions of lunar daytime? This question was asked 30 years ago, but it was never answered. Why not?
When the moon hits your eye like a big pizza pie - that's Amore!
That's exploration for you. Quite frankly, we really haven't looked that closely at a lot of things on Earth, much less other bodies.
This is quite surprising though. I'd think that spectrometers on other craft, or even orbiting telescopes, would have picked this up. Can we get some confirmation from some different instruments please?
@Robert: And tick off the moon goddess? No way!
The scientists' excuse for not discovering this sooner is that the Apollo moon soil and rocks were "bone dry."
But they were not bone dry. What actually happened was that
Samuel Epstein and Hugh Taylor published a series of papers in the 1970s in which they wrote "lunar water" in quotation marks because they were sure all the water in the samples was of terrestrial origin.
The explained samples with relatively low water content by stating that they were "a result of more careful handling." Samples with "unusually high water content" were completely dismissed by saying the water was brought to the moon by the astronauts and the rocks were very water absorbent.
So we spent $150 billion on Apollo (in today's dollars) and we never knew the moon samples were full of water because Epstein and Taylor put quotation marks around "lunar water?"
What else was missed in the Apollo moon rocks?
jack: the embargo was broken yesterday, at 3 minutes to midnight by a newspaper in India. as soon as that happened, everyone -- from bloggers to the NYTimes -- published their stories about this. the embargo was then canceled. ethan is actually "behind the curve" on this.
Jack and Grrl,
Even with the embargo broken, I still wanted to respect it. The two hours early publication was a surprise to me; I slated it to publish at the proper time, but I had an unanticipated technical issue which published it two hours early.
I'm actually fortunate that the embargo was cancelled, otherwise I'd be kicking myself, and I wouldn't be the only one!
So does this mean we also have deuterium?
If so we could use the He3 (is it still thought to be on the moon in extractable quantities?) we could power the moon with fusion.
I see. The post dated 11 am PT which is 2 pm ET. Sorry Ethan. I stand corrected.
This is quite surprising though. I'd think that spectrometers on other craft, or even orbiting telescopes, would have picked this up ! no away !
Doesn't this really give a boost to the moon base concept? Seems like hauling water from Earth would be a huge logistical problem. Of course sucking a liter of water out of a cubic meter of rock would probably not be a cake walk either.. In any case, this is way cool!! (and thanks for using my picture, I'm honored!)
I once stared at the moon with a multichannel imaging radiometer - but since I was on the earth I could only claim to see evidence of water in the earth's atmosphere. Still, I was surprised that I could actually see into space with the thing; I was expecting the water in the atmosphere to hide everything - then again I could see the sun so I guess I shouldn't have been too surprised to see the moon.
I forgot to say: visit grrlscientist's blog (see link in Ethan's post) and vote to send her to the frozen wastelands of the south. :) Quick ... there is only a day or two before the competition closes.
This find does make me look at the moon differently. However, why did it take so long to figure this out and to think about this? We went from 'knowing' that there was no water on the moon, that it was impossible; to suddenly the moon is full of frozen water all over. What sparked this sudden interest and sudden findings? I may be skeptical but I can admit that this is an incredible find and a step up in understanding the moon and its resources.
I always thought it was funny that the Moon was supposed to be the only dry body
Is it just me or does 0.1% water by volume not sound like a whole heck of a lot.
At peak oxygen consumption a person in top fitness can use 6+ liters of oxygen per minute under full workload. Of course oxygen can be conserved by being less active, iirc under general anesthesia use drops to 200-300ml/min for most. So it seems that under worst case conditions, a person will use all the oxygen contained in the water of aprox. 1.5 metric tons of lunar soil in about 4 minutes. Again, iirc, the standard resting rate of a fit to very fit person is 5%-10% of peak, so at rest you could stretch the oxygen from one and a half tons of lunar soil and provide 40-80 person minutes of oxygen. I don't know physiology well enough to provide a better estimate of total oxygen used per day, but it seems you would need to process an absolute minimum of 36 metric tons of lunar soil per day per person, with a physical maximum of 540 metric tons. That seems like a lot of rock to process....
What confuses me about this is that the [water/hydoxyls] are on the surface in the moon morning. To me it seems like you are trying to get your daily water needs by combing the grass after a light dew - it's going to take acres of surface to get a cup of water.
"Moon" is looking more realistic all the time. Mooncrete, for instance, uses only a little water.
The main reason why scientists thought the Apollo samples were contaminated with terrestrial water was because the ratio of deuterium to hydrogen in the lunar samples was the same as the ratio in water on earth, and it was assumed that it would be impossible for moon water to have the same ratio as earth water, therefore the so called "lunar water" must have come from earth.
Now they are saying that the isotope ratio on the moon really is the same as on the earth. Oops!
:D :D
ãIdeas are like the stars --- we never reach them, but like mariners, we chart our course by them.
"Is it just me or does 0.1% water by volume not sound like a whole heck of a lot."
No. That's A WHOLE LOT. It means, that it's feasible to extract it for rocket fuel.
"At peak oxygen consumption a person in top fitness can use 6+ liters of oxygen per minute under full workload. Of course oxygen can be conserved by being less active, iirc under general anesthesia use drops to 200-300ml/min for most."
Oxygen is not a problem, we can regenerate it easily (in rebreathers). Also, you can extract it from variety of minerals (some of them contain more than 10% of oxygen by weight).
Good post, Ethan. Note that detection of water all over the Moon and not just in the permanently shadowed craters was expected since the solar wind bombards the surface with protons that bounce around the surface and can combine with oxygen in the rocks. Some volatiles, including water, from impacting comets and asteroids likely made contributions to the water and OH budget we're starting to see there. Clemtine and Lunar Prospector data suggested this widespread result with their detection of hydrogen about a decade ago, but it's really cool to finally have confirmation that the hydrogen is actually bound to oxygen. This makes the upcoming LCROSS impact even more exciting!
Alex Besogonov @24: Ah! If you are hunting for lightly bound hydrogen rather then an oxygen source this news seems better. It was quotes like
that confused me. I'm not totally sure about space applications in specific but I thought that all re-breathers capture the CO2, and VOCs, add O2 to make up for use, and send the rest back to the person breathing. Having a source of hydrogen to make water from available oxygen must be nice as well. While you lose ~28 percent volumetric efficiency, and have to re-crack the water, you no longer have to deal with a cryogen prone to turning organics into high explosives.
Go India! I hope we eventually land a man on the moon, now that would make me immensely immensely proud!!
I don't know physiology well enough to provide a better estimate of total oxygen used per day, but it seems you would need to process an absolute minimum of 36 metric tons of lunar soil per day per person, with a physical maximum of 540 metric tons. That seems like a lot of rock to process....
This is awesome! For real! This actually means that there is chance that there might be some bacteria on the moon right???
wew.. water on moon? so far i've known, moon attracts the earth's water or as it called gravititional pull and it would not absorb earth's water :-)
@denparser
haha.. i think so.
Which is great. Thanks for nice post.
This might explain the odd properties of regolith slopes and drill holes. I think all our measurements were done on bone dry stuff, and that's why they could never recreate the "drill experience" described by the astronauts.
When the original "moon rocks" were analyzed no water was expected and none was found. Everyone knew the moon is a dry place and any water would soon be lost by the lack of an atmosphere and high temperatures of the lunar day. But now we know that there is significant water on the moon. The question remains why wasnt water found in the original "moon rock" samples. Why did the "moon rocks" show evidence of being formed on a planet with a spinning liquid magnetic core? Were the "moon rocks" dessicated and processed earth rocks? Was the lunar landing faked?
http://scienceblogs.com/gregladen/2009/01/the_moon_had_a_spinning_liqui…
To believe that the moon once collided with the earth and left a chunk of itself here is not to far out there. The ice melting being pulled by gravity spreading, stirring, atoms colliding our roaring ocean violent and strong.We see the moon day to day as we were before our atmosphere was created by the collision. Say the moon was a meteor that struck and upon collision started themselves into a spin breaking off the chunk of moon and then spinning amorously around in a gravitational pull for 4 million years.Creating a rare atmosphere. The trapping of gases the water allows is truly the rewards for us. We are not the center just the chance that it could happen. Could be seen as the ultimate space fuel. Water?