"Electricity can be dangerous. My nephew tried to stick a penny into a plug. Whoever said a penny doesn't go far didn't see him shoot across that floor. I told him he was grounded." -Tim Allen
I know what you're thinking. "Of course I know what static electricity is!" Oh, really? Let's go through the basics.

You've all (hopefully) gotten to play with a Van de Graaff Generator at some point. It's one of the simplest electricity demonstrations there is. You stand on something like a milk crate, touch your hands to the generator, have someone turn it on, and your hair (for those of you with hair) stands up on its end!
The reason for this, of course, is that when you turn the Van de Graaff Generator on, the top of it charges up (with positive charges). If you're connected to it, then you charge up with positive charges as well.
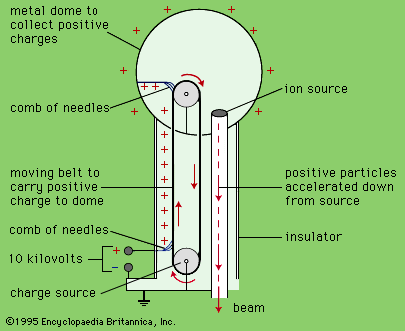
Since positive charges repel one another, those of you with straight enough, long enough hair will notice that the electric forces in your hair easily become more important than gravity or any other electric forces. This causes the very fun phenomenon of causing your hair to stand on end, since positive charges repel other positive charges.

Now, what you're used to calling static electricity is a little different. You probably think of rubbing two objects together, like your socks on the carpet, or a piece of glass with some silk.
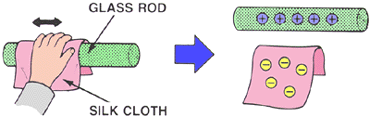
And, as you were (properly) taught, one of these materials loses electrons, leaving it positively charged, while the other one gains electrons, leaving it negatively charged.
This applies to a whole bunch of things, like rubbing a balloon against your hair.

After a good static charging, you'll notice that the balloon can do all sorts of interesting things: cause your hair to stand up, stick to the wall, or annoy the ever-living-daylights out of your poor grandpa.

How does this happen? Presumably, you've stolen some electrons from the balloon leaving it positively charged. And when you bring it close to a neutral object -- like a wall -- you attract the "opposite" charges on the wall (the electrons) and repel the "like" charges (the nuclei). As long as this configuration remains, the balloon will remain stuck to the wall, as the electric forces, due to static electricity, will hold it in place.

And that's how you were taught static electricity works.
Turns out, that picture is not quite right. Why not? Imagine what should happen if you take two identical materials, like two sheets of office paper.

If you rubbed them together, you'd expect that neither one would wind up with a static charge on them, right? They're made of the same material, so neither one should give up negative charges to the other, and so there shouldn't be any charge.
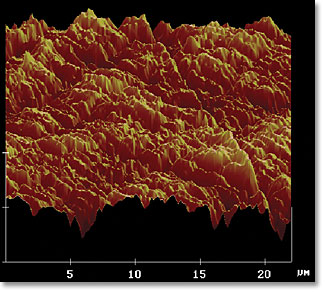
Only, that's not what happens. Let's take a closer look at this sheet of office paper.
Smooth as paper might seem, at a microscopic level there are tiny imperfections on the surface, visible in the image above. When you take two of these sheets of paper (or any two identical materials) and rub them together, what do you suppose happens to the voltages on the surface? Amazingly enough, no one had done this experiment until this past year! But thanks to Professor Grzybowski's group at Northwestern University, we now have the results from this, and they are spectacular. (Ars Technica writeup here.)
Instead of not charging, they totally pick up static charge! In fact, different sections of each surface pick up large amount of positive or negative charge. What we've been looking at, this whole time, as static electricity, is just the net charge on these objects. But what actually causes the individual molecules to attract or repel a nearby object has very little to do with the overall charge and everything to do with how those particular nearby molecules are charged! To put it in the authors' words:
For centuries, it has been assumed that such contact charging derives from the spatially homogeneous material properties (along the material's surface) and that within a given pair of materials, one charges uniformly positively and the other negatively. We demonstrate that this picture of contact charging is incorrect. While each contact-electrified piece develops a net charge of either positive or negative polarity, each surface supports a random "mosaic" of oppositely charged regions of nanoscopic dimensions. These mosaics of surface charge have the same topological characteristics for different types of electrified dielectrics and accommodate significantly more charge per unit area than previously thought.
So yes, some materials gain electrons and other materials lose electrons when you rub them together. But it is now thought -- and this is brand new -- that every statically charged material has significant regions of both positive and negative charge!
Not only is this a new finding, it's now thought that this is the dominant reason for static electricity.

But don't worry. You can still be "static cling" for Halloween. Only this time, you'll know how it works!


So, in effect they are saying that it is an organization of charge within each opposing member even if the material is of like quality and better seen if the material is of a differing quality.
It really does pay to revisit what was once accepted as good enough, now that we have better tools, we can see a deeper reality...cool!
Keep up the good work Ethan,and thank you.
I've enjoyed your post and the multitudes of intelligent commenterâs.
(FM static)Take me as I am
http://www.youtube.com/watch?v=Sl6T8mByII8&feature=fvst
I take my leaveâ¦
âAs I awoke in the middle of the night, I stepped outside to be greeted by a photonic cadence of fireflies set against the dark silhouette of a nearby stand of trees as they mimicked the milky way and the distant stars and galaxies, I then realized the we are not made of normal matter nor is it to be considered ordinary, we are unique matter in a Universal time where we can still behold distant massâ¦stars our distant cousins.â
Cheers
Sphere Coupler
What happens when a statically charged object is grounded by a spark? That looks like a big unipolar discharge which would suggest that the charged object is not just patches of positive and negative charge but also has an overall charge imbalance.
Awesome -- this seems like it has the potential (heh) to open a whole new subfield of physics, or at least a subsubfield. I wonder if the 'mosaic of charge' model is analogous in any useful way to magnetic domains.
Wow, I never thought I'd learn something new about something so "elementary". Great write up!
I presume that this effect is more pronounced in non-conducting substances (like paper) than in conductors like (say) aluminum foil...
Thanks, like always useful.
@csrster
As was said, there's still a net charge imbalance. It's just that net charge is distributed unevenly across the surface.
This is what causes fresh reams of paper to jam in the printer or copier, no? And fanning the ream before putting it in dispels the charge?
ha! smarty pants scientists are wrong! for centuries! makes me wonder what else they are wrong about?
hmmm...evolution...relativity...AGW...geocentrism...that face on mars...homeopathy...tiny rocks...african swallows...
actually, this is very cool. ZapperZ posted about this on June 26. it is an excellent example how science is self correcting. theories can change as new evidence is uncovered.
@lynxreign, #8:
I always assumed that was due to the cut fibers on the edges interlacing with each other between sheets?
@10 Doug:
I always assumed that the problem was caused by air pressure and a relative absence of air between the sheets. Fanning work because you introduce air into the space between the sheets, reducing or eliminating the vacuum.
Yep, time once again to update the STATIC ELECTRICITY MISCONCEPTIONS website. Sheesh. It never ends.
But finally this explains a long standing mystery: why do soft shiny plastic sheets cling to glass electrostatically, yet the surfaces end up with ~zero net charge when peeled apart?
.
Hmm, one piece of data and we are ready to toss out the old? Seems like this is one I'm waiting to see again, and again, and then again. Or do I misunderstand? Why would like charges aggregate? Chemists have been trying to make multicharged gas phase clusters for years - it ain't easy.
Oxidation products? Does the effect take place in the absence of free oxygen or are they defining oxidation as the loss of electrons?
BS
That's not Van der Waals force?
BS
Anyone who has ever worked in an office knows that paper can build up a charge against another piece of paper.
Perhaps scientists should talk to secretaries more often. >.>
Julie, were the papers delivered by a machine, such as a copier? Because sheets of paper delivered by rollers or belts in industrial settings are known to develop large charges.
BS
Best demonstration of static electricity I ever saw was when we did emergency communications for the RI Red Cross. When we got into the space it was still storming. We noted someone had conveniently cut the PL-259 connectors off the coaxial cables. But they left some conductor exposed.
Those conductors were sparking like crazy. And we knew it was static electricity from the wind whipping over the vertical antenna.
Would the process become less and less effective the larger the area of contact would be?
The charges can't move about (static electricity and no conductor), therefore when you place them nearby, the larger the area, the lower the proportion of areas that line up to an appropriate charge.
Or is this where the charge-sorted surface is brought to a generally neutral surface? In that case, the ability to cling would increase with the scale size of the regions of similar charge on it (which may depend upon the conductivity of the material).
Of course.
Thanks for the writeup with pictures. I was taken aback when I first read about this new discovery, but the pictures help explain it to my friends.
How did you get that shot of Newt Gingrich with balloons stuck to his head?
Met the guy once long before he went completely loony tunes. He really does look like that.
Hmm, one piece of data and we are ready to toss out the old? Seems like this is one I'm waiting to see again, and again, and then again. Or do I misunderstand? Why would like charges aggregate? Chemists have been trying to make multicharged gas phase clusters for years - it ain't easy.
Hmm, one piece of data and we are ready to toss out the old? Seems like this is one I'm waiting to see again, and again, and then again. Or do I misunderstand? Why would like charges aggregate? Chemists have been trying to make multicharged gas phase clusters for years - it ain't easy.
Essentially like materials or not, in our simplest forms all we are is positive and negative charges. Them elements that were made of is no different. just like a sign wave in ac. building collapsing. I wonder if you could create the same affect using static electricity?? Would be a good project.. just thinking out loud
If static electricity were sparked through a 12V automotive coil, would that coil lower the voltage and raise the amperage? Could that converted electricity, now with amperage, be directed into a battery for later use? Do this enough times and perhaps fully charge the battery. Pulse charging, is what they all it, I think. Attach an inverter and use resulting AC for whatever at a later point?
If the above is correct, maybe windmills that drive a Van De Graff Generator in low wind conditions could produce usable electricity, since Lentz's Law wouldn't apply for magnets and coils, as with most wind turbines.
Or, perhaps a static electricity collector (long Ham radio antenna) could collect enough to consistently fire off through an automotive spark plug and into an automotive coil and charge a deep cycle batteries in a home battery bank or an electric car.
Also, an antenna like this might be put on the exterior of an electric car to add range, since it's pretty windy outside a moving vehicle, which would charge that static electricity collector during transit.
Maybe static electricity really isn't what we once thought it was. Perhaps it is a clean, abundant, renewable, energy source that is all around us, just waiting for us to figure out how to put it to use in our lives.
WOW,that was amazing. I had never thought that a balloon would stick on a wall.
Electricity is a huge part of your existence that you can as smartly be one of the most few people who take into account the gadget of presents and fees to which we're so indebted.
ESD is a myth, I have never seen it nor have you. Next you'll be telling me what fire is made of!