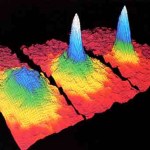
Cold Atom Tools
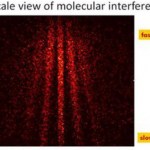
I know I said there weren't going to be physics posts for a while, but yesterday our Communications office passed along a media request about this paper on feedback cooling of BEC, from some sort of communications-person mailing list. I'd seen it talked up elsewhere-- here, for example, so I banged out an email to the reporter in question. Who didn't use any of my stuff in the story that ran late last night.
Having put in the work, though, I may as well get something out of it, so here's the email I sent. Questions in bold are from the original request. The paper is in the New Journal of…
Through some kind of weird synchronicity, the title question came up twice yesterday, once in a comment to my TED@NYC talk post, and the second time on Twitter, in a conversation with a person whose account is protected, thus rendering it un-link-able. Trust me.
The question is one of those things that you don't necessarily think about right off-- of course an atom is a particle!-- but once it gets brought up, you realize it's a little subtle. Because, after all, while electrons and photons are fundamental particles, with no internal structure, atoms are made of smaller things. But somehow we…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
Element: Sodium (Na)
Atomic Number: 11
Mass: one stable isotope, 23 amu
Laser cooling wavelength: 589 nm
Doppler cooling limit: 240 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it's a greyish metal at room temperature. Like the other alkalis, it's highly reactive, and bursts into flame on contact with water. For this reason, all physicists working with sodium have True Lab Stories about accidentally blowing stuff up with it.
Other properties of interest: Scattering length of around 80 a0; Feshbach resonance at around 900 G.
History:…
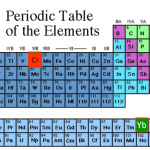
At the tail end of the cold-atom toolbox series, I joked about doing a "trading card" version shortening the posts to a more web-friendly length. In idly thinking about this, though, it occurred to me that if one were going to have cold-atom trading cards, it might make more sense to have them for the atoms, rather than the techniques. And having just devoted many thousands of words to technique, I don't really feel like trying to cut those down more, but atoms...
The "featured image" up top is a slide from my laser cooling lectures for our first-year seminar class. Elements outlined in red…
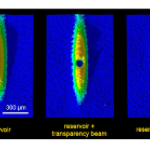
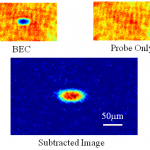
This is probably the last trip into the cold atom toolbox, unless I think of something else while I'm writing it. But don't make the mistake of assuming it's an afterthought-- far from it. In some ways, today's topic is the most important, because it covers the ways that we study the atoms once we have them trapped and cooled.
What do you mean? They're atoms, not Higgs bosons of something. You just... stick in a thermometer, or weigh them, or something... OK, actually, I have no idea. They're atoms, yes, but at ultra-low temperatures and in very small numbers. You can't bring them into…
In our last installment of the cold-atom toolbox series, we talked about why you need magnetic traps to get to really ultra-cold samples-- because the light scattering involved in laser cooling limits you to a temperature that's too high for making Bose-Einstein condensation (BEC). This time out, we'll talk about how you actually get to those ultra-cold temperatures.
What do you mean? I assumed it was just part of the trapping process? No, because the forces involved in magnetic trapping are like those involved in optical dipole traps. In physics jargon, they're "conservative" forces, which…
We're getting toward the end of the cold-atom technologies in my original list, but that doesn't mean we're scraping the bottom of the barrel. On the contrary, the remaining tools are among the most important for producing and studying truly ultra-cold atoms.
Wait, isn't what we've been talking about cold enough? There is, as always, more art than science in the naming of categories of things. "Cold" and "ultra-cold" get thrown around a lot in this business, and the dividing line isn't quite clear. Very roughly speaking, most people these days seem to use "cold" for the microkelving scale…
Today's dip into the cold-atom toolbox is to explain the real workhorse of cold-atom physics, the magneto-optical trap. This is the technology that really makes laser cooling useful, by letting you collect massive numbers of atoms at very low temperatures and moderate density.
Wait a minute, I thought we already had that, with optical molasses? Doesn't that make atoms really cold and stick them in space? Molasses does half the job, making the atoms really cold, but it doesn't actually confine them. The photon scattering that gives you the cooling force and Doppler cooling limit produces a "…
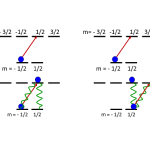
This topic is an addition to the original list in the introductory post for the series, because I had thought I could deal with it in one of the other entries. Really, though, it deserves its own installment because of its important role in the history of laser cooling. Laser cooling would not be as important as it is now were it not for the fact that cooling below the "Doppler limit" in optical molasses is not only possible, but easy to arrange. That's thanks to the "Sisyphus cooling" mechanism, the explanation of which was the main reason Claude Cohen-Tannoudji got his share of the 1997…
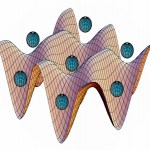
Last time in our trip through the cold-atom toolbox, we talked about light shifts, where the interaction with a laser changes the internal energy states of an atom in a way that can produce forces on those atoms. This allows the creation of "dipole traps" where cold atoms are held in the focus of a laser beam, but that's only the simplest thing you can use light shifts for. One of the essential tools of modern atomic physics is the "optical lattice," which uses patterns of light to make patterns of atoms.
OK, what do you mean "patterns of light"? Well, remember, light has both wave and…
The last post in this series on the core technologies of cold-atom physics dealt with optical molasses, where you use the scattering of light to exert forces on atoms to make them very, very cold. It turns out, they end up even colder than the simple theory would lead you to expect, which is very surprising, but also essential to the revolutionary impact of cold atom physics. If you were stuck with the Doppler cooling limit temperatures, laser cooling probably wouldn't be as big a deal as it is now.
You can do better, though, thanks to the interaction of several bits of physics that go beyond…
`Once upon a time there were three little sisters,' the Dormouse began in a great hurry; `and their names were Elsie, Lacie, and Tillie; and they lived at the bottom of a well--'
`What did they live on?' said Alice, who always took a great interest in questions of eating and drinking.
`They lived on treacle,' said the Dormouse, after thinking a minute or two.
`They couldn't have done that, you know,' Alice gently remarked; `they'd have been ill.'
`So they were,' said the Dormouse; `VERY ill.'
-- Lewis Carroll, Alice's Adventures in Wonderland, Chapter 7
As an undergrad, I did my senior…
This series of posts is intended to explain the tools and tricks used to create and manipulate samples of ultra-cold atoms; thus, it's appropriate to start with how we get those atoms in the first place. This will be a very quick background on the basic force used to make atoms cold, and then the technology of atom sources for a variety of experiments.
Okay, so you've got two things in the post title. Which are we going to talk about first? Well, the study of cold atoms really begins with the observation that light can be used to push atoms around. There are actually two ways to do that, but…
I have a small collection of recent research papers that I'd like to write up open in various browser tabs and suchlike, but many of these would benefit from having some relatively clear and compact explanations of the underlying techniques. And while I can either dig up some old posts, or Google somebody else's, it's been a while since I wrote some simple, straightforward explanations of physics techniques, so I thought it'd be fun to write up some new explanations for use in future posts. Thus, this introduction to a series of techniques commonly used in my corner of Atomic, Molecular, and…