Science
"Education is an admirable thing, but it is well to remember from time to time that nothing worth knowing can be taught." -Oscar Wilde
As many of you know, last weekend I launched a suggestion box here on the site, and I've been overwhelmed by the response: about fifty of you have sent something in to me in the first less-than-a-week of this alone!
Image credit: Thao Nelson of http://mycredo.wordpress.com/.
So, let's start answering them! There are more than enough excellent questions and suggestions to keep me busy for a long time, but with the new academic year starting up, one of…
The stupid Steven Pinker business from a few weeks ago turned out to do one good thing after all. It led to this post at Making Science Public, which quoted some books by Jacob Bronowski that sounded relevant to my interests. And, indeed, on checking The Common Sense of Science out of the college library, I opened it up to find him making one of the arguments of my book for me:
Many people persuade themselves that they cannot understand mechanical things, or that they have no head for figures. these convictions make them feel enclused and safe, and of course save them a great deal of trouble…
Two chapters of the book-in-progress will be devoted to the development of the modern understanding of the atom. One of these is about the Bohr model, which turned 100 this year, but Bohr's model would not have been possible without an earlier experiment. The actual experiment was done by Ernest Marsden and Hans Geiger, but as is the way of such things, the historical credit mostly accrues to their boss and noted force of nature, Ernest Rutherford. This is the experiment that established the cartoon image of an atom as a solar system, which is utterly unworkable using classical physics, and…
I started out blogging about books, way back in 2001, but somewhat ironically, I rarely post anything about books any more. My free time has been whittled down to the point where book blogging is time taken away from other stuff, and it's never been that popular here. I post reviews of science books that publishers send me, but fiction has mostly fallen by the wayside. As has fiction reading, to a large extent.
I did have a run recently of books with some elements that resonated with my own book-in-progress about scientific thinking, so I figure that's a good excuse to bring them in. The…
Element: Cesium (Cs)
Atomic Number: 55
Mass: One stable isotope, mass 133 amu.
Laser cooling wavelength: 854nm, but see below.
Doppler cooling limit: 125 μK.
Chemical classification: Yet another alkali metal, column I of the periodic table.
This one isn't greyish, though! It's kind of gold color. Still explodes violently in water, though.
Other properties of interest: The definition of the second in the SI system of units is in terms of the microwave transition between hyperfine ground states in Cs-- 9,192,631,770 oscillations to one second, to be precise. Has a really large scattering…
I don't know how I missed this one, given that it's over two weeks old, but I did. Since yesterday was a holiday in the US and I had done a long post the day before because something that happened on Friday had really irritated me, I figured I might as well take a stab at this because it represents one of my "favorite" quack apologists at his most over-the-top quackiest. More importantly, it won't take too much brain power to deconstruct, but could be entertaining nonetheless. I'm referring, of course, to Mike Adams, the "Health Ranger," of NaturalNews.com. Of course, nothing by Mike Adams is…
Recently I blogged about historians of science who chronicle scientific debates of the past neutrally and leave it to the reader to find out who (if anyone) turned out to be right in the end. This approach pisses me off because I'm a scientist and I believe that the main point of such debates – past and current – is to advance science. I don't enjoy the implication of neutralist history of science, that it's all just historically contingent talk and the process isn't taking us anywhere.
Historian of science Darin Hayton of Haverford College in Pennsylvania didn't like my viewpoint and wrote…
It's been a banner week for blogging advice, between John Scalzi's thoughts on comments and Bee's advice on whether to write a science blog. Both of them are worth a read, and I don't have a great deal to add, but writing the stuff I'm supposed to be writing this morning is like pulling my own teeth, so throwing in a couple of brief remarks is much more enjoyable.
First, Bee's introduction raises an important issue:
I used to think there should really be more scientists blogging. That’s because for me science journalism not so much a source of information but a source of news. It tells me…
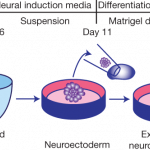
While we've been waiting and waiting for the physicists to get their act together and deliver on Mr Fusion home energy sources and flying cars, the biologists have been making great progress on the kinds of things that turn biologists on. The latest development: growing tiny little human brains in a bucket. Only let's not call them brains…they are cerebral organoids. Hugo Gernsback would be so proud.
Here's the latest development. Start with embryonic human stem cells, or induced pluripotent stem cells (cells which you've reset to a kind of embryonic state by using a virus to transfect them…
Element: Chromium (Cr)
Atomic Number: 24
Mass: Four "stable" isotopes between 50 and 54 amu. Chromium-50 is technically radioactive, with a half-life considerably longer than the age of the universe, so...
Laser cooling wavelength: 425nm, but see below.
Doppler cooling limit: 120 μK.
Chemical classification: Transition metal, smack in the middle of the periodic table. Shiny.
Other properties of interest: Has a fairly large magnetic moment in its ground state, 6 Bohr magnetons, which means it has strong magnetic interactions. For this reason, it's kind of an interesting system to study-- you…
I've been revising a chapter on collaboration in science for the book-in-progress, making an analogy to team sports. And it occurred to me as I was trying to find a way to procrastinate, that while science is a highly collaborative endeavor, most of the popular stories that get told about science are not. There's no Hoosiers of science out there.
Now, admittedly, the sample of great pop-culture stories about science period is pretty small. But what does exist mostly concerns individual struggles-- the lone genius who can revolutionize science by just thinking about it in isolation, but who…
Element: Lithium (Li)
Atomic Number: 3
Mass: Two stable isotopes, masses 6 and 7 amu
Laser cooling wavelength: 671 nm
Doppler cooling limit: 140 μK.
Chemical classification: Alkali metal, column I in the periodic table. Yet another greyish metal. We're almost done with alkalis, I promise. Less reactive than any of the others, so the explosions in water aren't very impressive.
Other properties of interest: Lithium-7 is a boson, but has a negative scattering length, meaning that BEC's of lithium-7 tend to implode unless you modify the collisional properties. Lithium-6 is a fermion, and much…
Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it's presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around…
In one of those Information Supercollider moments, two very different articles crossed in my social media feeds, and suddenly seemed to be related. The first was this New York Post piece by a college essay consultant:
Finally, after 15 or so years of parents managing every variable, there comes the time when a student is expected to do something all by herself: fill out the actual application. Write an essay in her own voice.
By this point, our coddled child has no faith in her own words at all. Her own ideas and feelings, like a language she has not practiced, have fallen away.
Her parents…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
Adam Frank has an op-ed at the New York Times that tells a very familiar story: science is on the decline, and we're living in an "Age of Denial".
IN 1982, polls showed that 44 percent of Americans believed God had created human beings in their present form. Thirty years later, the fraction of the population who are creationists is 46 percent.
In 1989, when “climate change” had just entered the public lexicon, 63 percent of Americans understood it was a problem. Almost 25 years later, that proportion is actually a bit lower, at 58 percent.
The timeline of these polls defines my career in…
When I wrote up the giant interferometer experiment at Stanford, I noted that they've managed to create a situation where the wavefunction of the atoms passing through their interferometer contains two peaks separated by almost a centimeter and a half. This isn't two clouds of atoms each definitely in a particular position, mind, this is a wavefunction representing a bunch of atoms that are each partly in two places at the same time , separated by 1.4 centimeters.
I emailed Mark a link to the post, and in his reply he said that they've increased that to about 4cm (which is just a matter of…
Element: Xenon (Xe)
Atomic Number: 54
Mass: nine "stable" isotopes, masses from 124 to 136 amu. Xenon-136 is technically radioactive, but with a half-life of a hundred billion billion years, so, you know, it's pretty much stable.
Laser cooling wavelength: 882 nm
Doppler cooling limit: 120 μK
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with anything, so poses much less danger to scientists than any of the alkalis, though it has, at times, been used as an anesthetic, and Will Happer's group at Princeton has a funny story about a student's…
After a couple of very productive days where I closed my Twitter tab because it was too freakin' annoying to read, I checked in briefly Wednesday morning, and found Rhett Allain and Frank Noschese discussing this Veritasium bullet-in-block experiment:
Tom at Swans On Tea offers some analysis, and Rhett offers a video response doing out some of the math:
I basically agree with their explanations, and that should have been that, except that in his discussion Rhett mentioned that the bullet probably doesn't go as far into the spinning block, which prompted Frank to ask whether you could…
Element: Helium (He)
Atomic Number: 2
Mass: two stable isotopes, 3 and 4 amu.
Laser cooling wavelength: 1083 nm
Doppler cooling limit: 38 μK
(It should be noted, though, that despite the low temperature, laser-cooled helium has a relatively high velocity-- that Doppler limit corresponds to an average velocity that's just about the same as for sodium at 240 μK. This is because temperature is a measure of kinetic energy, and helium is much, much lighter than any of the other laser-cooled elements.)
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with…