"I have this one little saying, when things get too heavy just call me helium, the lightest known gas to man." -Jimi Hendrix
Hendrix, as I told you once before, was almost right. We know of helium, conventionally, as the lighter-than-air gas that we fill balloons, blimps and zeppelins with in order to quickly and easily "defy gravity" here on Earth.
At least defying gravity is what it appears to do. But what's really going on is that helium is simply a very low-density gas. Our atmosphere, a mix of mostly Nitrogen (N2) and Oxygen (O2) gases, has an average molecular weight of 29. With a molecular weight of four, Helium is bested only by pure Hydrogen (H2) gas (with a molecular weight of 2) in the low-density department.
And just as a stone sinks in the ocean or olive oil floats atop a glass of water, the principle of buoyancy ensures that the least dense materials will eventually -- once the effects of mixing are negligible (which they are, for atmospheric gases, over long enough times) -- find themselves layered atop the progressively more dense materials.
This is true for solids (such as in the interior of the Earth, where the core is denser than the mantle, which is denser than the crust, which is denser than the oceans and so on), liquids (as shown above), and gases as well. For a gas giant like Jupiter, because it's so massive (many hundreds of times the mass of Earth), it's gravitational well is tremendous, and even the lightest of its atmospheric gases stay bound to the planet itself. On the other extreme end, the Moon and asteroids are far too low in mass to hold on to any of their atmospheric gases, and even the heaviest gas will be expelled from the planet thanks to the intense radiation coming from our Sun.
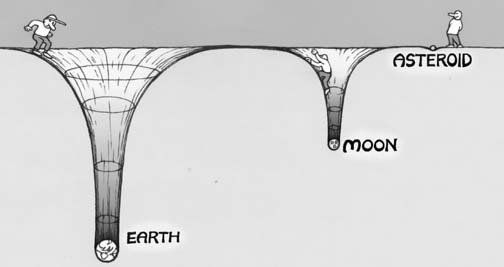 Image credit: retrieved from http://clowder.net/hop/railroad/asteroids.html.
Image credit: retrieved from http://clowder.net/hop/railroad/asteroids.html.
The Earth's atmosphere is today mostly composed of Nitrogen (78%), Oxygen (21%), with trace amounts of other gases. However, if we could go back to when the Solar System was first forming, we'd discover that every planet would have had an atmosphere loaded up with the two most common elements in the Universe: Hydrogen and Helium.
The vast majority of the Hydrogen and Helium would have been confined to the Earth's atmosphere, and quickly would have risen to the uppermost layers. Other atoms -- Carbon, Nitrogen, and Oxygen -- would have bonded with Hydrogen (which is reactive) and not with Helium (which isn't) to produce methane, ammonia and water/water vapor. While the pure hydrogen gas and the helium would have been kicked off the planet relatively quickly by the Sun, the remaining gases would have stuck around for much longer, eventually leaving behind only the heaviest components of our atmosphere.
Now, it's no problem to "make" hydrogen, we've got oceans full of material that's 2/3 hydrogen. But what about Helium?
From the birth of the Solar System, there's practically no Helium left on Earth. (To be more precise, Helium composes 0.00052% of the Earth's atmosphere by volume.) But there is plenty of Helium here on Earth, and it has nothing to do with Helium anyplace else in the rest of the Universe.
 Image credit: Rich Olson of http://nothinglabs.blogspot.com/.
Image credit: Rich Olson of http://nothinglabs.blogspot.com/.
These are some radioactive samples of Uranium and Thorium, two of the rarest elements found in nature. The most common isotopes -- Uranium-238 and Thorium-232 -- are radioactive, and will undergo a decay chain over timescales of billions of years.
Billions of years. In other words, a timescale comparable to the age of the Earth. Elements with much shorter lifetimes have all decayed away; that's why Uranium (or maybe Plutonium) is the heaviest naturally occurring element here on Earth; while many others were certainly around immediately following the supernova explosion that triggered the formation of our Solar System, everything with shorter lifetimes is completely gone by now. But when Uranium-238 and Thorium-232 decay, they do so in a very particular and important fashion.
They undergo a process known as alpha decay, where heavy elements emit an alpha particle, eventually leading to the creation of a more stable nucleus. But the "alpha particle" is actually the nucleus of a Helium atom! It will very quickly (in a matter of microseconds) pick up a couple of electrons and become a neutral helium atom, and since Helium atoms don't react with anything, all they'll do is attempt to rise up to the top of the atmosphere.
Which they can only do if they can find the atmosphere! Most of the Uranium and Thorium in the world exists deep underground, so wherever there's a Uranium or Thorium deposit in the Earth's crust that's been there for a long time (like, hundreds of millions of years), you're bound to find a large deposit of Helium gas, too. Luckily (perhaps) for Earth, the largest one in the world sits squarely underground near the middle of the United States.
But we don't replenish this Helium! We mine it, but once we mine what's there, it will take either hundreds of millions of years to make more, or we need to find a new source of Helium, such as through mastering terrestrial nuclear fusion or perhaps mining the Moon.
In the meantime, we should be aware that every time we fill a Helium balloon, we're taking something that it took the entire natural history of the Earth to create and basically banishing it from our planet.
Helium is a rare and unique element, with some amazing scientific properties. It doesn't become a liquid until it's cooled to a temperature of 4 Kelvin, and it becomes a superfluid with some fantastic, frictionless properties when it's maybe half that temperature.
As long as we don't conserve and recycle our Helium, we are dooming mankind to a future shortage of Helium, and each balloon we fill with it (or inhale, for you teenagers) means that much less Helium for future generations here on Earth. We only discovered this element less than 150 years ago, and the U.S. National Helium Reserve is expected to be empty by 2018, with the remainder of the Helium supply completely privatized.
I'm not here to scare you into not using Helium balloons or to convince you that we need government regulation of Helium production or consumption; I'm simply telling you where Helium comes from, and why we're going to run out of it if we keep doing things the way we're doing them. I've done my part by telling you the science behind our Helium; the rest is up to you.








This is crazy! I had no idea we were using up our helium reserves... why does the human race overall not concern themselves with our mass consumption problem?
I read things about helium and how the price is way out of whack to where it should be. In other words, ridiculously expensive. The question is, why isn't it? Heck, I have a small tank in my basement I bought for a party for twenty bucks. I guess I'll save it til 2018, then eBay!
And just as a stone sinks in the ocean or olive oil floats atop a glass of water, the principle of buoyancy ensures that the least dense materials will eventually — once the effects of mixing are negligible (which they are, for atmospheric gases, over long enough times) — find themselves layered atop the progressively more dense materials.
So, how did an unreactive gas like CFC, 4+ times heavier than air, manage to get up high enough into the upper atmosphere in the 1990s to create the scarifying ozone layer holes in the polar regions?
Alan
1: CFCs are a catalyst. That means it is still there to do its thing again
2: Heavier gasses are still available up there, just in very small amounts. 10% at 1km, 1% at 2km, .1% at 3km, etc. still leaves some at 50km.
3: "Long enough timescales". You quoted it but didn't consider it.
4: The statement is about LIGHTER molecules and atoms. Not heavier ones. CFCs in the stratosphere aren't going to leave. He will.
Alan, the lower atmosphere is turbulent, gases get mixed regardless of density. Really high you up you get some density separation, but a better explanation is that the lighter the molecule, the higher it's thermal velocity. If you take a light atom at the top of the atmosphere like hydrogen or helium, there is a small probability that it will reach escape velocity and leave the Earth, while heavier atoms will fall back. This is helped by the solar wind.
Incidentally, we are very lucky that we have a cold layer at the tropopause which almost no water vapor can pass without condensing and falling down as rain. Water that gets above that point can dissociate by UV, and the hydrogen can escape. If more water reached higher into the atmosphere or more UV-radiation could pass lower, our oceans would have dried out by now.
What's that? Is it. Hmm. And what to do?
They say that earth could run out of Helium in 25 to 50 years.
They've said that about oil to; yet the known reserves keep increasing. Discovery.
Oh well, what do we do?
Ahh, first someone needs to set up a Helium Futures Market, trading Helium as a commodity like pork bellies or gold or oil.
But that may take a while.
In the meantime, go to your local cylinder gas company and buy a few cylinder of helium and stockpile the stuff.
I know, give your kid a cylinder of Helium as a stocking stuffer for Christmas. It's only $125 for a cylinder that can fill 175 9" balloons.
But don't fill any balloons. If the shortage is real and no new Helium is discovered; you'll be able to sell that cylinder of Helium in 20 years and pay for an ivy league college education!!
disclaimer #1: I am not sane (hmm that might be misconstrued)
disclaimer #2: no guarantee, warranty or representation, express or implied, is given as to the accuracy or completeness of the above Helium investment advise
disclaimer #3: Oh wait a minute; don't buy that Helium cylinder; it's a bad investment
"Most of the Uranium and Thorium in the world exists deep underground, so wherever there’s a Uranium... you’re bound to find a large deposit of Helium gas, too."
Look at this http://en.wikipedia.org/wiki/List_of_countries_by_uranium_reserves
I get it . No I don't get it.
Hmm? If you get it tell me.
Wait a minute. Look at that link Australia is loaded with Uranium.
And Nambia and Niger have more Uranium than the US. That's not right! Bring on the Helium wars!
Alan: In addition to the points Wow and Thomas made above, CFCs can be broken apart by UV photons. This results in free chlorine atoms, which are what do the damage to the ozone layer. The reactions are Cl + O3 -> ClO + O2 and ClO + O3 -> Cl + 2 * O2. Rinse and repeat. The chlorine atoms will eventually mix out of the stratosphere, but that takes decades, and in the meantime each chlorine atom can destroy hundreds if not thousands of ozone molecules.
O3 existing there because of the same UV light and that interception being why O3 is a necessary shield against UV damage to organisms (e.g. us).
I hate helium balloons.
They waste helium for frivolous purposes. They pollute the environment and kill animals for frivolous purposes.
So many of them float away -- either on accident when a little kid lets go of them, or even more released on purpose like in that image in the article -- and end up somewhere far away where a bird or other animal either eats them or gets tangled in the string and dies.
Stop using helium balloons!
I'm seeing a Bond Villain here. Hans Zeetwo? Dr. Noblegas? Monopolizing the world's helium and then waiting for it to become expensive/critical seems like a very bond villain thing to do. Frivolous, yes, but it might even raise some awareness.
On a more serious note, this may be part of a triple whammy coming this century. We are already starting to feel a pinch in medical radioisotopes, because not many places have realistic plans for replacing/building new research reactors. And at some point in the next few decades, the demand for rare earths for high-tech applications may outstrip what China is willing to export.
great stuff Ethan, this will be interesting. Are there are any thoughts on how much helium there might be on the moon?
eric: Rare-earths aren't that much of a problem. When China decided to limit exports, that only caused other mines that had closed because of China under-cutting their prices to re-open. There's a long-term issue with rare-earths, but the proximate issue is only a temporary pain.
Helium is also used as the 3rd-stage coolant for imaging equipment in hospitals, as that equipment has to be brought down to 4K for the superconducting magnets to do their stuff.
Liquid Nitrogen is used as the second stage. Standard refrigeration as the first.
The slightest little accidental addition of energy to the liquid helium sheath can cause a bubble, and that bubble causes a chain reaction as ALL the Helium boils off.
The equipment includes an emergency vent which lets out all the Helium if this happens in order to protect the equipment from exploding.
If you ever get a chance, make friends with somebody in Radiology and get them to give you the tour!
copernicus34 The talk about helium on the moon is He-3, an isotope that might be useful in fusion and that would them be worth the extremely high price of collecting it. .
It would be easier to gather up the helium that is found in our atmosphere. This is some orders of magnitude more expensive than today, but doable. We wouldn't get helium balloons, but we could have refrigeration like the one Vince describes, assuming some better recycling of the helium.
I actually had a similar thought but just the opposite. assuming we get fusion reactors working within the next decade or so we will be converting mass amounts of hydrogen into helium, every country every continent. we will be converting hydrogen from water to helium, which is of little industrial use. i imagined a day where we were worrying about the amount of helium we were releasing into the atmosphere and the effects that would have on the planet. while the helium may just evaporate off and leave us little worry we never get it back. regardless of the power source we find and how clever we think we are it's never renewable and we need to think of the long term results.
Helium can be distilled out of air with cryogenic equipment, so we will always be able to produce it for critical applications, it just won't be cheap enough for balloons anymore.
Also a lot of helium is produced as a byproduct of drilling for natural gas. The helium that comes from that is pretty much on a "use it or lose it" basis, we can't store it for later. This serves to make helium even cheaper than if we had to intentionally drill for the stuff.
@Thomas, thanks for the info. Yeh its kinda what i was thinking, awfully expensive (at least now) to try and acquire it on the moon. Are there any thoughts as to how much might be there though? I've yet to read anything pertaining to educated guesses on that.
Ethan, this is way off topic, but I can't find a way to contact you directly.
I've got an MP3 I want to send you of a song recorded by a local trio that I think you will like.
Where should I send it ?
Would it be possible to mix hydrogen with another gas to give it similar properties to helium whilst making it non-inflammable?
Mix it with Oxygen. It puts out fires then!
...You'd have water bombs instead of balloons - good one!
Seriously though we've got get this frivolous usage under control - they ain't making any more!
This isn't so much a "science" problem but rather one of economics... the allocation of a scarce resource.
In a free market the price of any scarce resource is directly proportional to it's scarcity. The more scarce, the more costly. As supplies of He dwindle the price rises and consumers will either pay more and lessen consumption (seeking substitutes) and/or suppliers will find or invent new economical ways to recover the decreasing marginal supplies.
A perfect example are petroleum resources. Not long ago we were hearing tales of "end of oil." Rising prices incented the development of new recovery technologies such as fracking that have pushed "the end" of recoverable oil supplies into the very distant future.
Sure, distortions occur but they are usually the result of policy rather than free-market dynamics.
The other place you find He is in Gas fields. Only in very small amounts but it is there. Qatar, one of the worlds major exporters of LNG, is one of the worlds main sources of Helium. In a few years they'll be the 2nd largest supplier in the world.
It is only cost effective because when making liquid natural gas the percentage of He is increased. When the price rises enough I suspect other gas companies will start extracting the He.
Glad the economists have it all worked out. Scarcity in one resource leads us to expend other, seemingly less scarce resources to recover more of it at greater cost. For instance, with fracking we can exchange relatively unpolluted fresh water, habitable property, wildlife habitate, and arable farmland for some more oil. We can do this indefinitely, for a definition of "we" and "indefinitely" that goes something like this:
"All humans who survive into the future will survive into the future with whatever standard of living people who survive into the future retain."
That this makes economics out to be a discipline that is as useful to anyone who gives a damn about humanity as the study of ballistics is to anyone who is concerned about gun violence. When triggers are pulled, bullets will fly. This is the "law" of bullets and triggers. When people want bullets to fly, but can't find any more triggers to pull, they will simply find something else to turn into a trigger, so that they continue to make the bullets fly. People who do not like the consequences will not like the consequences. Other people won't care. Some will be dead. Maybe many. Thus endeth the wisdom of whatsofuckingever it matters.
So is the helium just floating like a skin atop our atmosphere? Can we just scoop/skim it off and distill it?
I read somewhere the US govt is trying to sell off its helium dirt cheap, hadnt heard about the private part -- sounds like some conspiracy by helium vendor with big connections.. Maybe like debeers cornering tne diamond market, people will be swayed to give helium balloons as engagement presents instead of diamond ring
He man,
Nope, the helium does not just sit at the top of the atmosphere. The average velocity of helium atoms at temperatures ambient to the earth is greater than the earth's escape velocity. Therefore, the helium atoms escape from the earth's gravitational pull and wind up in space.
Does anyone actually mine helium? I thought it's a by product from natural gas production. If they do not go to the trouble extracting the helium from the natural gas, the helium is not going to stay in the ground. It would be released to the atmosphere when the natural gas is burnt.So, not filling a balloon with helium would not slow down (or speed up) the depletion of helium from the underground reservoirs. To have any effect, you need to slow down or speed up the extraction of natural gas.
I drill and process Helium for a living. You said "we mine it" that is incorrect. We drill for it, and it is generally found with Natural Gas in quantities less than 1%. The main reason why we are wasting helium is because it has been price controlled by the government, because during WW2 the government thought that dirigibles ie blimps were going to be the wave of the future for combat so they wanted to artificially control the price. It was classified a war time asset, why they haven't fixed it yet is just silly. Congress is currently working on rewriting a piece of legislation that will fix this and you will see the price of helium increase 20 fold. Whatever happens you can kiss your helium balloons good bye.
Another Republican disaster. Thanks, guys.
AC,
How is this a specifically Republican disaster. As Barton pointed out above, He is cheap because its price is kept artificially low due to its potentially military use during WWII. In case your education in history is deficient, there weren't many Republicans in the US government during that war. FDR was president, and certainly no Republican.
Though the two parties swapped places in the 60's or thereabouts, so your disassociation there is definitely not indicative of an error in AC's post.
I rather suspect it was a "strawman poe" where someone is attempting to get people to assert that this is what democrats "really think" and thereby neuter anyone who identifies as a democrat.
Wow,
My point was that I think we try to politicize things that aren't really political. I don't hold either Democrats or Republicans specifically responsible for the helium shortage. It's a bipartisan "F up".
Sean, my point was yours wasn't a rebuttal, and it was written to imply this is what it was.
It's a bipartisan F up because doing something on such a non-voter-issue could only go wrong and be noticed, since if it went OK, it wouldn't be noticed at all. Most of the people being voted are more interested in getting voted for again, hence Obama will not give all people in Gitmo that have not been found guilty as charged a pardon because he (well, his party) would be hammered in the pre-election smear campaigns for supporting terrorists.
I think they'd win more votes than they lose, but the other problem is that the politicians live in a bubble where they don't hear anything from the average Jenny Housecoat. So if the Washington pundits say it's not a vote winner, they will believe it.
oh no ! how can we survive without helium ??
For those folks who are planning on stockpiling that tank of helium left over from your last party; don't hold your breath. Helium, being an incredibly tiny, non-reactive molecule, seeps through just about any container you can put it in. Yes, even a steel tank. Over time, the helium will migrate through the steel, and your tank will go empty just like that sad, deflated balloon a week after the party. I have no idea how they hold it in the National Stockpile. Anyone care to enlighten us? (get it? en-LIGHTEN us?)