"In three words I can sum up everything I've learned about life: it goes on." -Robert Frost
Well, here we are, at the end of the week, and you know what that means: another Ask Ethan column! This week, I've been asked about the same thing by many different people, so I'll credit it to Patrick E. and Matt T., who want to know:
I'm curious to hear your take on this: the 2nd Law of Thermodynamics applied to the origin of life.
For those of you who haven't heard, this is what they're talking about.
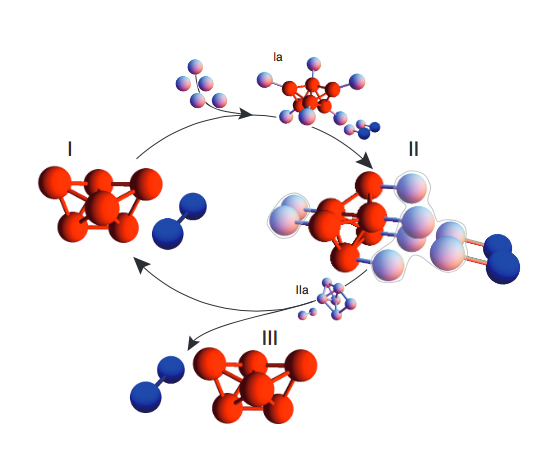
 Image credit: Richard A. Cunha, Thereza A. Soares, Victor H. Rusu, Frederico J.S. Pontes, Eduardo F. Franca and Roberto D. Lins.
Image credit: Richard A. Cunha, Thereza A. Soares, Victor H. Rusu, Frederico J.S. Pontes, Eduardo F. Franca and Roberto D. Lins.
We've long known that the Universe didn't start out with anything resembling life. Forget about complex, multicellular, highly differentiated creatures like mammals, angiosperms and insects; immediately after the Big Bang, the Universe didn't even have atoms in it!
But out of that incredibly hot, dense soup of primordial matter and radiation came everything. And for many of the steps along the way, we completely know how.
The earliest quarks-and-gluons, part of the primordial plasma of the Universe, became tightly bound as the Universe expanded and cooled, decaying into the protons and neutrons that we're more familiar with in short order. There are good reasons for this: energetically, it's more favorable to have quarks and gluons bound together in those configurations -- protons and neutrons -- than to have them in any other configuration.
When there's plenty of available, free energy, there's nothing compelling you to be in a more stable state, but as the Universe expands and cools, down the ramp you go, until you reach the bottom. And that same process continues.
Those protons and neutrons -- once the Universe cools to the point where photons don't immediately blast apart the things they combine into -- combine to form heavier, more stable atomic nuclei. If there were enough activation energy, they'd form even heavier, more stable elements at this time!
As it is, helium-4 is pretty much the practical limit at this time, but as the Universe continues to expand and cool, there becomes another way for the particles in the Universe to become more tightly bound: the atomic nuclei and electrons can bind together, forming neutral atoms for the first time.
 Image credit: Ned Wright (and possibly Will Kinney) from Ned Wright's cosmology tutorial, via http://ned.ipac.caltech.edu/level5/Sept02/Kinney/Kinney3.html.
Image credit: Ned Wright (and possibly Will Kinney) from Ned Wright's cosmology tutorial, via http://ned.ipac.caltech.edu/level5/Sept02/Kinney/Kinney3.html.
Of course, they can only do this once the Universe is cool enough; if you have too much energy, the neutral atoms will be blasted apart back into ions and electrons, but if the ambient radiation/temperature/particle energy is below the binding energy of electrons to nuclei, you'll get neutral atoms.
And you notice a theme here: some processes happen spontaneously, where once the energy of your surroundings drops below a certain threshold, things just wind up bound in lower energy states, and some processes require a certain amount of "activation energy" to climb over a threshold, resulting in a more stable state overall. Let's go a little further.
After gravitation binds the Universe together into clumps -- again an example of a spontaneous "binding" -- the most overdense regions accumulate enough matter at high enough temperatures and densities that nuclear fusion ignites, producing heavier and heavier elements. This, again, is an example of requiring an "activation energy," but in the end, the products you wind up with are more tightly bound (overall, once everything is averaged together) than the reactants you started with.
And after the heaviest of these stars go supernova, the interstellar medium becomes heavily enriched with all the atoms in the periodic table, and they bind together in a variety of ways to create molecules: simple ones at first, but eventually some incredibly complicated ones as well.
What I want you to keep in mind is that, at every step along the way, we've gone into a more energetically favorable configuration, and there were only two general ways we went there: either we spontaneously "rolled down an energy hill" to get there, or we "rolled up an energy hill and then down into a lower valley" to get there.
That's it. In all cases, the energy reactions looked like one of the following curves, to some extent.
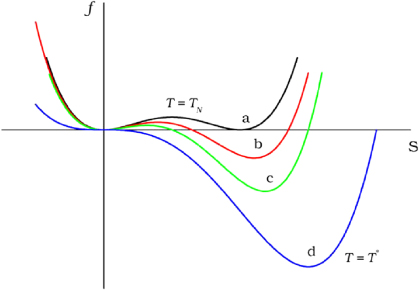 Image credit: Sindhunil Barman Roy 2013 J. Phys.: Condens. Matter 25 183201. doi:10.1088/0953-8984/25/18/183201.
Image credit: Sindhunil Barman Roy 2013 J. Phys.: Condens. Matter 25 183201. doi:10.1088/0953-8984/25/18/183201.
So, then, what about that big step? We can go from subatomic particles to atomic constituents, from protons, neutrons and electrons to atoms, from atoms to all the elements of the periodic table, from all the elements to a huge diversity of molecules, but at some point, we have to take that leap. You know the one I'm talking about: where non-life turns into life.
It's pretty well accepted that either life originated very early on in Earth's history, or perhaps even before that, in the cosmos somewhere prior to that. Once we have life, Darwinian evolution -- mutation, limited resources and natural selection -- can definitely get us from those first, self-reproducing organisms to the full breadth and diversity of life we see on the planet today.
But it's not a fundamental explanation; it doesn't tell us the mechanism behind how or why these changes occur, or what the physical process is that powers them.
It's hard, in many ways, to draw the line between life and non-life. Those of you who grew up around the same time I did might be surprised at how well the old Sesame Street definition still holds up today.
 Image credit: Kenneth Libbrecht of http://www.its.caltech.edu/~atomic/snowcrystals/.
Image credit: Kenneth Libbrecht of http://www.its.caltech.edu/~atomic/snowcrystals/.
You don't need life for that; crystals are certainly not alive, but from rock to ice, crystalline structures self-reproduce.
Quite interestingly, so can sphere clusters on a molecular level.
And perhaps even more surprisingly, so can vortices, a simple example of fluid flow!
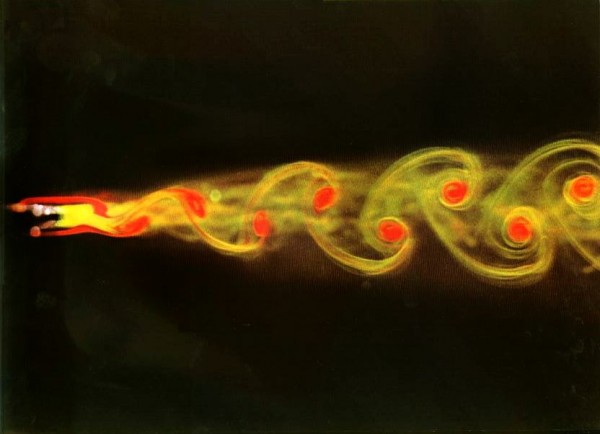 Image credit: On the Flow of Fluids Made Visible, Henri Werlé, Leonardo, Vol. 8, No. 4. (Autumn, 1975), pp. 329-331.
Image credit: On the Flow of Fluids Made Visible, Henri Werlé, Leonardo, Vol. 8, No. 4. (Autumn, 1975), pp. 329-331.
None of these things are alive, though, in the way we think a virus or bacterium is alive. But Jeremy England, a physicist at MIT, thinks that the same physical principles -- very similar to what's led us along every known step of the journey thus far -- might be responsible for all of these things.
(The complete -- and eminently readable -- paper is here.)
It's all based on energetic favorability, something a physicist is likely to simply call thermodynamics. The basic idea is that if you take a bunch of "whatever" -- atoms, molecules, etc. -- and you apply a low, variable amount of energy to them over time, they're going to wind up shaking down to a more tightly bound state. This is the same way a gentle-but-inconsistent shaking of a jar of mixed nuts will eventually leave the largest nuts at the top and the smallest nuts down at the bottom: it's a more energetically stable configuration.
Here's the thing: what England says is definitely physically true. It's also almost certainly not the entire story of life. For two examples, evolution doesn't always (or arguably, even often) find the most energetically favorable solution to a problem.
We've never evolved wheels, even though that would be hugely more efficient for a variety of applications. Yet no animal has ever evolved it.
And all the plants in all the history of the entire world have always relied -- as far as we know -- on the same two molecules for gathering and storing energy from the Sun: Chlorophyll A and Chlorophyll B. These are not the only molecules capable of storing solar energy or even of permitting photosynthesis, as there are bacteria that use other molecules to achieve it. The solar spectrum even peaks in the green/yellow, while Chlorophyll A and B both absorb blue and red light, but reflect green/yellow!
But just because that one phenomenon doesn't govern everything doesn't mean it isn't the phenomenon responsibly for the origin of life; it might be. But it's very speculative at this point. As Harvard professor Eugene Shakhnovich says,
"Jeremy’s ideas are interesting and potentially promising, but at this point are extremely speculative, especially as applied to life phenomena."
There are plenty of applications of thermodynamics -- and of an increased entropy of the environment -- to biology and to life processes, but does life emerge, causally, on account of entropy and thermodynamic phenomena?
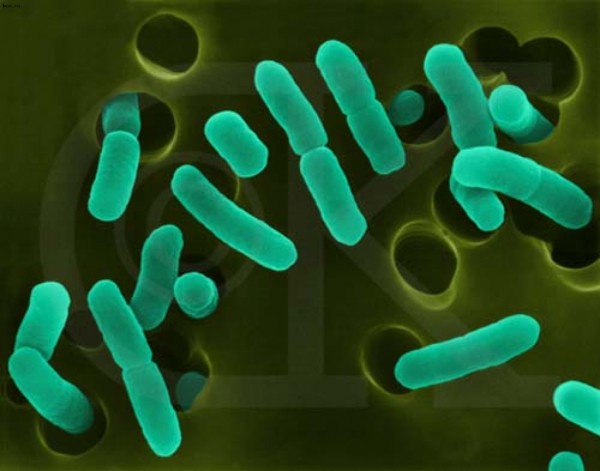 Image credit: E. Coli bacteria, retrieved from http://scitechdaily.com/bacteria-replicate-closely-to-the-actual-thermo….
Image credit: E. Coli bacteria, retrieved from http://scitechdaily.com/bacteria-replicate-closely-to-the-actual-thermo….
It's possible, but the jury is not only still out, they likely won't be back for a long time. This is an incredibly difficult hypothesis to test, and we very likely won't believe we have the answer until we've achieved the holy grail of abiogenesis: creating life from non-life for ourselves! So we still don't know why or how life exists, but it's conceivable and reasonable that thermodynamic processes may hold the answer to that. It's also very cool that people have been not only researching this for decades, but that the research is leading us down all sorts of interesting paths, some of which might have something to do with the origin and evolution of living (and almost living) things here on Earth!
And that will wrap up another Ask Ethan for this week. Have a question or suggestion for what you’d like to see us tackle next? Ask away! And if you haven't been over to the new Starts With A Bang at Medium this week, you've missed out on nine new fantastic articles this week alone; better catch up!






So here's my Ask Ethan question triggered by this blog post:
you said (as all do): "after the Big Bang, the Universe didn’t even have atoms in it!
But out of that incredibly hot, dense soup of primordial matter and radiation came everything. "
If there are not even atoms, thermometers, etc. Why is it said to be 'incredibly hot?'
Kenny: Because temperature isn't something you need a thermometer to measure. "Temperature" is just a synonym for "average kinetic energy", and kinetic energy is just a synonym for "speed at which mass is moving". As long as the components of the universe are moving (be they quark/gluons, atoms or molecules) then they will have a temperature that can be quantified from the average speed of that motion. No thermometers or atoms required.
@ Kenny
Another perspective;
A) The universe has been continually expanding and cooling. We know that. Now just extrapolate backwards. It's gonna be really hot and compressed, amazingly so at some point.
B) With the help of particle colliders, we know what energy levels (aka temperatures) it takes to momentarily turn BARYONS (protons/neutrons) back in to their constituent quarks. Were talking WAY HOT!
Extra: That hellish plasma also included lots of MESONS (quark-antiquark pairs held together by gluons) which annihilated one another in the midst of that early primordial ruckus. Only the BARYONS escaped the infernal battle unscathed in large numbers
DISCLAIMER (thanks to Ken Ham): I was not there.
@ Max & @MandoZink -- One thing that's never been clear to me is that that very hot state also needs to be a state of very low entropy (for time to work right). But I always associate "way hot" with high entropy. Where am I wrong?
Thank you for bringing us this topic Ethan, Why Life Exists. The research that you point to Statistical physics of self-replication
Jeremy L. England is absolutely excellent research. This paper is mostly readable and the summary article that Ethan points to in a couple of places is very readable.
So very nice to read a new important idea in understanding how life arose.
There are very few wheels among living things. Bacterial flagella are the best example. Problem with wheels is that they work well only on a smooth substrate. That is why there is so much effort put into designing walking robots and other vehicles by the military. There are some animals which will roll up into a ball and roll down a slope, (I don't recall which ones) sort of wheel like.
@ Jim Thomerson
True rotary locomotion only evolved in some higher reptiles, as seen in this photo of the North American Hoop Snake (Serpentus circulus):
http://www.mentalfloss.com/wp-content/uploads/2007/09/MFhoopsnake.JPG
Apparently this capability is still evolving as either parallel or convergent evolution, as seen in these wanna-be rollers:
http://www.baanmaha.com/community/thread16693.html
There is little support that the imitators (second example) have ever accomplished any significant rotation-based displacement, but anecdotal evidence lends strong and convincing support for for the Hoop Snake's speed and endurance during various lengths of transit.
Once again, I was not there.
"There are very few wheels among living things. Bacterial flagella are the best example. Problem with wheels is that they work well only on a smooth substrate"
Flagella work in water. Not noted for being rough surfaced...
"“Temperature” is just a synonym for “average kinetic energy”, and kinetic energy is just a synonym for “speed at which mass is moving”."
Well, more accurately it's "Bulk internal motion".
Thanks for this! This is quantitative work derived directly from earlier work on non-equilibrium thermodynamics (NET).
And it ties nicely in with Russell et al work on alkaline hydrothermal systems and how such disequilibrium system as life arises out of them due to NET. However, they argue convincingly (to me) that the metabolic bottleneck isn't disequilibrium and dissipation as such, but the increased dissipation that comes from positive feedback in "Atwood engines" of dissipating free energy (disequilibrium) flows (free energy conversion, FEC, engines).
More precisely, the simplest such FEC engine of electron bifurcating metal atoms that we still see in the core enzymes of the metabolic UCA. ["Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road", Branscomb and Russell, Biochimica et Biophysica Acta (BBA) - Bioenergetics, Volume 1827, Issue 6, June 2013, Page 806.]
That work also complement Ethan's description in the form of geophysics of terrestrial planet formation with differentiation and subduction cells leading up to the alkaline hydrothermal system dissipation, all engines of disequilibrium converging on the geochemistry one that we are distant cousins to. I assume that the initial disequilibrium is the same that is necessary for baryogenesis, and it would indeed be a result of local (vacuum) energy minimum. (At speed-of-light traversing domain walls of electroweak symmetry breaking, IIRC how Wkipedia describes it, travelling faster than the baryon-radiation soup can equilibrate, I take it.)
The thermodynamics of replicators may or may not supplement the metabolic achievement, but the result favoring RNA primacy is suggestive so far.
And here I always claimed that entropy has nothing to do with life as such, since the entropy produced by evolution during selection at each generation is minuscule compared to the entropy produced during organism growth. And if snow flakes can grow, so can cells. But England turns that around to face me. =D
@Kenny: "If there are not even atoms, thermometers, etc. Why is it said to be ‘incredibly hot?’"
There are fundamental particles that achieve a temperature (since the universe locally expands slower than light speed). And so there is an excellent "thermometer" there, a radiation thermometer of black-body radiation. We still see a later relic in the form of black-body microwave background radiation. You can read of the temperature from the radiation distribution (see Wikipedia on black-body radiation).
@ Patrick: Entropy of the universe is still very much discussed AFAIK. It is the quantity that makes least classical sense in my opinion so I wouldn't worry about it as compared to how to get a disequilibrium (free energy) of the universe as Ethan describes.
But FWIW one solution (which indeed puts early entropy as "low") is to count "holographic" microstates, making it related to quantum field and string theory both. [Linkweaver et al.]
But I always associate “way hot” with high entropy. Where am I wrong?
Entropy is a measure of how many different microstates a system has. It doesn't directly depend on temperature. However, it is true that a fluid will have more available microstates than a solid, because the atoms in a solid are generally confined to certain lattice points while in a fluid they are free to move.
Entropy frequently appears paired with temperature in the equations of thermodynamics. In SI the units are joules per kelvin.
I'm inclined to believe that the tipping point is where complex chemical reactions become dissipative systems, feeding on ambient energy-flows to increase their complexity. Then at some point those reactions become self-reproducing, and that's where you cross the line to "life."
All neatly driven by thermodynamics and chemistry, and then biology itself emerges and becomes a driver as well via Darwinian mechanisms.
The key to producing life in the lab is to keep experimenting with dissipative reactions until we hit one that keeps going and then starts reproducing itself. Easy to say, hard to do.
One of the hints I think will come from the abnormal proteins called "prions" that cause bovine spongiform encephalopathy (BSE, "mad cow disease") and related diseases (Creutzfeld-Jacob, possibly others). BSE proteins have the same chemical composition as normal proteins but have an abnormal "folding" or geometric configuration.
Normally we think of bacteria as life and viruses as almost-life or as the most primitive form of life. Viruses reproduce by attaching to cells and inserting viral genetic material to hijack the cells. BSE prions reproduce by attaching themselves to normal proteins in brains, and transferring their abnormal folding configuration to the target proteins, turning them into additional BSE proteins that go on to do likewise to normal proteins.
In effect this is a more primitive form of "information transfer" than that which is used by viruses. Instead of transferring molecules, they transfer what can be considered "geometry," something that's further down the scale of simplicity from molecules, and closer to "pure information." In that way, prions can almost be considered as "memetic replicators," if one considers "information" to be the core aspect of "memes."
So if we look for molecules that behave in this way, transferring "pure information" such as a geometric configuration, to other molecules, that might give us some clues as to the boundary between complex nonliving dissipative structures and rudimentary life.
Or this is "not even wrong" for some obvious reason I missed, and y'all can have a good laugh;-)
---
So now I'll stick my neck out and say "teleology."
Much further up the scale of complexity, life evolves brains that produce individual consciousness, and sufficiently complex consciousness has purpose & meaning, which is to say, telos. The naturalistic answer to supernatural or animistic beliefs that involve purpose as a property of the universe itself, is that once the universe evolved brains with minds, it produced teleology: Ours is not a teleological universe as such, but a universe that allows for teleology to exist. Seems to me this is a potentially useful meeting-point of common ground between those who ask "why?" and those who ask "how?"
"The key to producing life in the lab is to keep experimenting with dissipative reactions until we hit one that keeps going and then starts reproducing itself. Easy to say, hard to do."
http://www.gizmag.com/first-plastic-artificial-cell-working-organelles/…
Correction/edit: "...sufficiently complex consciousness has _a sense of_ purpose & meaning..." To explicate that a little further:
We have goals and we act to achieve our goals. That's purpose.
We "mean something" to those who are close to us, and they "mean something" to us. Very often we have a deeply-felt sense of personal meaning in relation to something larger than ourselves: knowledge, nature, nationality, a sports team or a performing artist, a personal or collective mythos, or whatever. Those things are meaning.
These purposes & meanings aren't inherent in the universe at-large, but they are inherent in ourselves.
Re. Wow at #14: Yeah I saw that news item in another venue. Very interesting. I'm not sure how conclusive it is, and I haven't followed up on the news item yet (this weekend was primarily spent doing engineering for a client).
A synthetic cell is still a cell, more complex than rudimentary life. But it may very well give us a path toward assembling the constituent parts in different ways, or working our way back from a cell to the precursor of a cell, and so on.
So the search for the boundary between nonlife and life, can be gotten-at from both sides, working toward the center.
I've gotta scoot now; be back tonight.
Heh, I love that you linked the old Sesame Street song, which I remember from childhood. "Breathe and eat and grow." I once asked my parents if that meant fire was alive, too. They didn't know how to answer. I figure fire is more like cancer, which is also alive, but destroys the organism.
Creating synthetic life, new organisms never before seen on planet earth, is being done. http://www.telegraph.co.uk/science/7745868/Scientist-Craig-Venter-creat…
In this TED lecture, Venter explains how he created synthetic life. http://www.ted.com/talks/craig_venter_unveils_synthetic_life.html
But of course, this is NOT creating life from scratch, so to speak. Venter needs to start out with a living cell in which he places new DNA programming and voila, a new species of bacteria.
"But it may very well give us a path toward assembling the constituent parts in different ways, or working our way back from a cell to the precursor of a cell, and so on. "
Pretty much how I see it.
The production of an evolved process may not make sense to an engineer, but if we can get a complex machine and then find ways it can be built up, we can investigate how THOSE can be created.
And having done that, work our way back through the plausibilities.
We have to make a story of what went on, but life doesn't DO stories, it does what it must, WE put stories on that.
Then when we've seen how these things can have come from machines too simple to be called "life", we can look at plausible paths reality on earth took to do something similar.
It strikes me that living things are purposeful. Water runs downhill because of physical laws. Salmon use physical laws to swim upstream to spawn.
@ Jim Thomerson
that's a keen observation. Indeed, there seems to be a level of "awareness" and purpose as you say, weather feeding or reproduction, in all that we deem "alive". Where does this come from is the question. We sadly can't mind meld with animals or plants. If we could "know" what i.e. a worm knows or in other words maybe, how much mentally "alive" it is, we would be much closer to the answer.
Wow @ 19: Agreed, life does what it must, and we put stories on it. As in, photons exist at different wavelengths, and we put color on that.
Jim @ 20: Agreed, living things are purposeful. At even the simplest levels, life seeks to live. The more complex the organism, the more it exhibits "behavior" rather than "reactions." An amoeba reproduces by fission: it just "happens" and doesn't appear to require the amoeba to "do anything" to initiate it. At the lowest level of sexual reproduction, an organism just "happens" to bump into another, without any obvious "searching-for" behavior, and they exchange genetic material: that also appears to be a reaction rather than behavior. When you get to the level of a paramecium, it appears to engage in "search-for" behavior to find another with which to reproduce. Further up the scale, increasingly-complex "search-for-mate" behaviors.
The same case applies to organisms' uptake of information from their environments: from simple homeostatic responses, to increasingly complex "learning" behaviors.
Sinisa @ 21: Here's a potential path forward. Per Stuart Hameroff, a paramecium exhibits "learning" behavior in being able to escape from capture by a capillary tube, increasingly quickly each time it occurs: yet a paramecium has no neurons. Hameroff's theory is that information processing occurs in proteins in the cytoskeleton, which would explain that behavior, and more to the point, explain how neurons can act as processing nodes in neural networks such as brains. The precursors of "awareness" appear to exist in even the simplest organisms that have capacity to process and act on information from their environments (again, behavior vs. reaction).
If Hameroff is correct (and so far the testable hypotheses derived from his theory are being empirically supported), then at some point it should become possible to "know what a worm thinks" as far as that's physically possible, by making testable inferences about the behavior of worms (and other organisms) based on new knowledge about the complexity of their information-processing capabilities. As with knowledge of insect eye optics, it gets us a bit closer to understanding how other organisms perceive and respond to their environments.
It would not be surprising to find that even relatively simple organisms have rudimentary awareness that they exist and feel various types of pleasure and pain. And that, in turn, would get us closer to the issue of "purpose" in simpler organisms.
"Water runs downhill because of physical laws. Salmon use physical laws to swim upstream to spawn."
However, looking at brain activity, you've made a decision before the bits that work out WHY you decide that activate.
When you feel pain and when you react are in the opposite sense to the one you insist is "true" yourself: your reaction comes before you realise that there's pain there, yet you insist that you felt pain and then reacted.
When the salmon rushes up stream, why? It's driven there by its instincts. If it WERE a choice, it could choose not to. It doesn't. So how much of a decision can it be?
I just had to share this amazing new research idea.
https://medium.com/the-physics-arxiv-blog/239bc4cf4ece
Life Could Have Evolved Just 15 Million Years After The Big Bang, Says Cosmologist
@OKThen
you posted this to again advertise Medium or...?? Because, if you wanted to link the paper, you could have done that...
Be that as it may, theory above just doesn't hold water. 15-20 million years is what is generally thought of as time needed for hydrogen clouds to collapse and form protosuns. Then they nova at some point, seed the space with heavier elements, then pass some more millions of years in order for first planets to form.
To have fully formed planets... with all the things needed to have life just 15 million years after BB is just wrong when you consider everything else. And by the way... what happens to that life once CMB drops below 30'C??
Can't have planets before you have stars. The period of 300.000 - 300.000.000 years after BB are called dark ages, for a reason. If there were stars, the neutral hydrogen would get reionized much sooner than what we detect...
I don't understand how a professor from Cambridge can have such a brainfart as this.
SL, black holes may have formed extremely early (see an earlier Ethan post on the subject), and the creation of these will often seed copious amounts of heavier elements.
I'd take the "15 million years" claim as a "speed of light" limit on how short it could have taken.
@Wow
Still, I feel temperature of 0-100C and possible BH is not enough to get planets and water and not get stars.
In order to make his theory work, one would first have to rewrite cosmic dark ages, put forth a mechanism of BH heavy element synthesis that produces enough heavy elements to actually make whole planets, water, and everything else (except massive stars, and lots of them, can't have reionisation). Even in 50 million years. The worse part is he only has a window when CMB is between 0-100'C (according to him), so that's what.. several million years....
Actually in the paper, he didn't do anything spectacular.. he just used simple zshift/temperature formula to get the time when CMB was worm. He didn't bother with fine details of how water and planets actually got there so early.
In the paper he uses the standard formula for collapse of gas to form stars. And then basically says woala... thus rocky planets could have formed... except he seems to forget that hydrogen/helium only gas is not the same thing as a planetary nebula. IMO he needs to show the evidence of planetary nebula in that time range first, before one jumps to making planets and running water.
ups.. typo
CMB is warm.. not worm :D
"except he seems to forget that hydrogen/helium only gas is not the same thing as a planetary nebula."
I think you're forgetting that black hole collapse often includes a supernova event that produces much more than hydrogen/helium.
That's true. But if those are the seed black holes, the first ones, which became supermasive ones when universe was just 1billion years old. They then "ate" their accompanying nebula anyway.
I don't know.. just reversing a clock based on cmb temperature and saying that "universe was habitable" when cmb was between 0-100'C is pretty .. well.. amateurish IMO. If this was a high-school paper, I would say wow, because at that level it shows inquisitive mind. From a cosmologist from Harvard/Cambridge... come on..
p.s.
i.e. if he showed a mechanism and calculation of how to get enough oxygen in those first 15 million years, and by which process they combine to create molecules of h2o. (where does the energy come etc..) Then show that they could be created in large enough densities to have water clouds that can collapse... I would be impressed and highly interested in this. Then I could believe that yes... it is not impossible that "some" life could have been made then (still taking production of amino acids etc... on a faith). Show me basis for life in those early times, and I can say.. it's possible. To make a point of temperature as the most important factor, and taking all else on "what if they are already there" is just useless. He can only say that Universe was 0-100'C 15 million years after BB. That's something any physics student can calculate.. and probably does. Don't feel that's paper material. But that's just MO