"I admitted, that the world had existed millions of years. I am astonished at the ignorance of the masses on these subjects. Hugh Miller has it right when he says that 'the battle of evidences must now be fought on the field of the natural sciences.'"
-James A. Garfield, future U.S. President, in 1859
You will find all sorts of ideas out there on how old the Earth, the Galaxy, and the Universe are. Some contend that it's only a few thousand years old, while others contend that it's infinitely old.

You and I are free to believe whatever we want, of course. But before you decide what you believe, consider this.

The easiest place to look to get an answer is at stars. One of the greatest scientific breakthroughs of the 20th century was figuring out what powered the Sun. And the answer is that, in the core of our Sun, the pressures, temperatures and densities are so high that individual atomic nuclei get fused together into heavier elements.
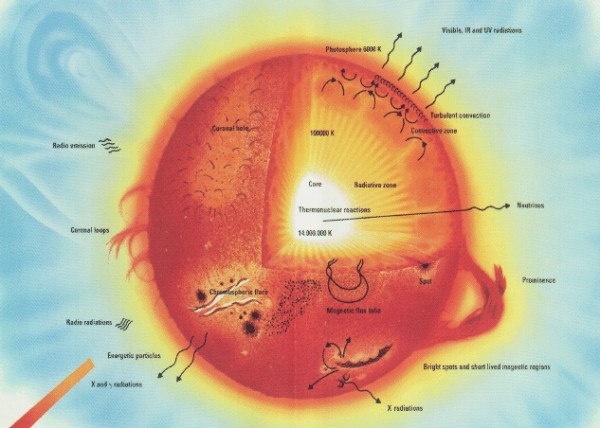
Specifically, the Sun fuses hydrogen, the lightest and most common element observed in the Universe, into helium, the second lightest element. It takes four hydrogen atoms to fuse into one helium atom, but when you measure the mass of helium, you find that it's just slightly lighter -- by about 0.7% -- that the original four hydrogen atoms. Where does that mass go?

It gets turned into energy, just like in a hydrogen bomb, by Einstein's famous E=mc2! Well, we look around the Universe at the others stars around, and it turns out that this is how most stars work: fusing hydrogen into helium.
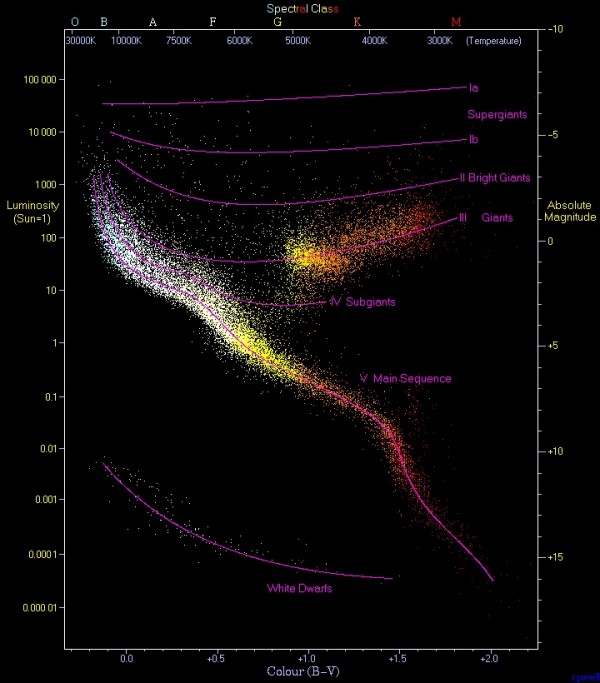
In fact, what we noticed is that if your star is fusing hydrogen into helium, there's a very close relationship between how bright your star is, shown on the vertical axis below, and what color your star is, shown on the horizontal axis and designated by the letters O B A F G K M.
See that big squiggly line labeled "V Main Sequence"? Those are the stars burning hydrogen into helium! There's also a whole slew of yellow stars connecting the main sequence to a brighter branch of stars, known as giants, which are what stars become when they run out of hydrogen and start burning helium into heavier elements!
But if we look only at the main sequence stars of these seven types -- O B A F G K M -- we can learn something very interesting.
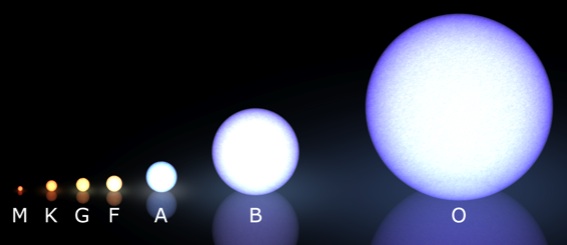
O stars are the most massive, while M stars are the least massive! In other words, if you're burning hydrogen into helium, being bigger will make you brighter and bluer, while being littler will make you dimmer and redder.
But there's one more piece to the puzzle. The bigger and brighter you are, the faster you burn up all of your fuel! In other words, the less massive you are, the longer it takes you to burn up all of your hydrogen, and therefore the longer it takes you to turn into a giant star and leave the main sequence!
In other words, by looking at what the least massive stars are that leave the main sequence, we can determine the age of a cluster of stars!
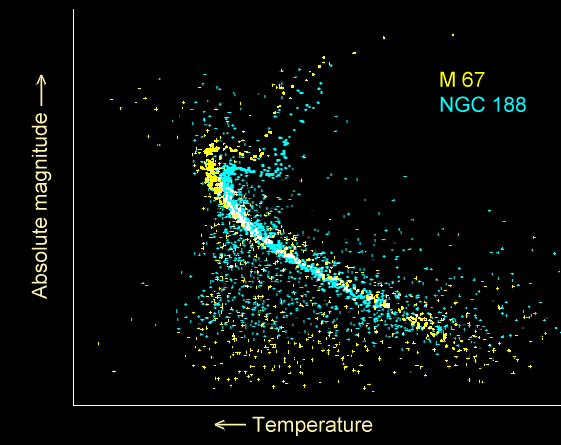
In the diagram above, there are two star clusters, M67 (in yellow) and NGC 188 (in blue). From where the stars "leave" the main sequence, we can conclude that M67 is about 4 billion years old, but that NGC 188 is just over 6 billion years old.
And if we do this for all the stars we can see in the Universe? We find that the oldest places in the Universe have only been around for 13 billion years, give or take a couple of billion.

The globular cluster, M15 (above), has no stars on the main sequence that are more massive than about 70% of the Sun! And while the Universe can certainly be as old as it wants, none of the stars in it show evidence of being older than around 13 billion years.
Just by looking at the stars in the Universe, it tells us that the Universe is at least 11 billion years old, and probably not much older than 13 billion years.
So when it comes to deciding how old the Universe is, I'm not going to tell you what to believe. But for my own part, I'm going to decide based on what the Universe tells us about itself, whether I like it or not.

Don't stars create a lot of He3? I seem to remember being taught that the difference between terrestrial and solar helium (besides location) is that terrestrial helium is much heavier in He4 since it mostly comes from the decay of U235. Please tell me if I'm wrong.
I like this method better than using the expansion of the universe to calculate when the Big Bang occurred. It's easier to understand (IMO) and isn't hampered as much by the question "What if the universe is significantly larger than the observable universe?"
I agree with science pundit that this approach is easier to understand than accelerating expansion, but I still wonder about what lies outside the light cone.
@The Science Pundit:
It is my understanding that a helium-3 nuclei fuses with a hydrogen-1 (protium) nuclei to get a helium-4 nuclei.
---
Reminds me of this:
- Carl Sagan
What stops stars that fuse hydrogen into helium from then fusing the helium immediately?
@4: Helium (at least 4He) is extremely hard to fuse.
The paired nucleons in the 4He nucleus form an extremely stable and tightly-bound configuration. In contrast, there is no stable configuration with 8 nucleons - if you bash two 4He nuclei together to get an 8Be nucleus, it decays straight back to two 4He almost instantly. Other reactions between 4He and other light nuclei are all either massively unlikely or result in products that either decay back to 4He or rapidly react with protons producing 4He again.
To get 4He to fuse you need to get three of them to collide within a very short time window (4He + 4He = 8Be, 8Be + 4He = 12C, the second reaction having to occur in the tiny window before the 8Be decays). Accordingly, this reaction requires far higher temperatures and densities than are needed for hydrogen fusion.
Why don't hydrogen-burning stars generate those temperatures anyway? Because in a stable main-sequence star, the core temperature has to generate the correct outward pressure to balance the gravitational pressure resulting from the star's mass; if the core gets too hot it expands and cools, decreasing the power output; if it gets too cool then it is compressed by the star's mass, heating it and increasing the power output. This self-regulation keeps the temperature stable as long as the star's composition doesn't change much; the change in composition as hydrogen is consumed is what eventually drives the star off the main-sequence.
Stars generate 3He as an intermediate product; further reactions (typically 3He + 3He or 3He + 4He + 1H rather than the more obvious 3He + 1H) consume the 3He and other intermediates, leaving 4He to accumulate.
The Big Bang generated a significant amount of 3He because it didn't last for long enough for other reactions to consume all the 3He, so there's a fair amount of it left, which probably accounts for almost all the 3He found in interstellar gas.
Terrestrial helium is indeed strongly depleted in 3He because virtually none of it is primordial; any 3He we find here has either been trapped since the formation of the earth (unlikely), arrived trapped in cosmic dust particles, or is the result of (usually artificial) nuclear reactions (such as the decay of 3H). In contrast we have a lot of 4He resulting from alpha decay (of lots of heavy nuclei, not just uranium).
"You" can't really figure out the universe this way. All you've done is asked the readers to take the diagram for the relationship between star color and age on faith, and no one who believes the universe is a lot younger will do that. You need to explain how you derive that diagram too.
Thomas, he did say that in the post, the time to burn up "all" of the hydrogen. (It goes back on stellar models, perhaps also cluster models, but that isn't a problem for the explanation of how the predictions are made.)
@4: Andrew @5 mentioned that there are no stable nuclei with eight nucleons. There are also no stable nuclei with five nucleons, so if 4He were to fuse with a proton or neutron it would spit the particle back out in a fraction of a microsecond.
There are two or three two-body reactions that might in principle produce lithium, but they must be rare (Li, Be, and B are all much rarer than other elements with a single digit number of protons), and all involve nuclei that are typically not available where a star is burning 4He. I don't know what temperatures are required; I'm not even sure the first of these can actually take place:
4He + 2H -> 6Li
4He + 3He -> 7Li + e+ + ν
4He + 3He -> 6Li + 1H
In the second reaction the extra particles are a positron (to dispose of the extra charge) and a neutrino (to conserve lepton number); these particles also carry off excess linear/angular momentum. These are the same extra particles that appear in the proton-proton reaction 1H + 1H -> 2H + e+ + ν. Once you have Li on hand, generating 9Be, 10B, and 11B should be straightforward. You don't, for all practical purposes, get any further along this path, since the three-body reaction 4He + 4He + 4He -> 12C is much more common, and from there the carbon cycle takes over:
12C + 1H -> 13N -> 13C + e+ + ν
13C + 1H -> 14N
14N + 1H -> 15O -> 15N + e+ + ν
15N + 1H -> 12C + 4He
To get 16O, you push another 4He nucleus into your 12C nucleus; you don't get this from the carbon cycle because it is energetically more favorable to let the 4He nucleus cart off excess linear/angular momentum. (Not all helium-burning stars will advance to the carbon-burning phase, which requires even higher pressures and temperatures. Similar processes, as well as collisions involving 12C and 16O, give you all of the elements up to 56Fe, which has the highest binding energy per nucleon of any nucleus. Going beyond iron requires a supernova.
The age of the universe like all time measurements is a relative thing. If a twin can go on a cosmic journey and come back 10 years younger than his stay at home twin; then in principle he can also go on a cosmic journey and come home 10 billion years younger than his stay at home twin.
So what then is this thing we call the age of the universe. In my mind, it is a Earth centric time that has pragmatic use in Earth centric astronomy. It simply means that this age of the universe is the delta time (all time is a delta, a relative to something else, not an absolute) that is the current maximum observable time.
A brief history of this measurement can be found here http://www.lhup.edu/~dsimanek/cutting/ageuniv.htm
I consider this chart to represent the sociology of the age of the universe. It is also a history of a particular physics measurement, delta time maximum.
But until this chart plateaus for some extended period of human earth science (maybe half a century); I think that we can only attach socialogical and historical meaning to the physical measurement "age of the universe"; but not physical meaning as in some particular "age" of the earth, of sun or of the milky way. The "age of the universe" has not stabilized enough; especially when we consider that the oldest stars in our milky way galaxy for example are essentially the "age of the universe."
Thus when the science of cosmology shows a plateau in the estimated "age of the universe"; then we will have an interesting physical quantity (perhaps even a constant of nature) to discuss.
@Thomas:
It's the age for a comoving observer - as usual in General Relativity. I. e. it's the age someone would measure who does not move with respect to the center-of-mass frame of the universe. One can measure if one is in such a frame by looking for the dipole in the CMBR (Doppler shift).
Since the Earth is *not* comoving, no, the time is not exactly the time as measured on Earth. However, since the total velocity of the Earth, the sun, the Milky Way etc. combined is only several 100 kilometers per second, this time is not much different from that measured by a co-moving observer - at most some 1000 years, I'd estimate.
You *did* notice that that page is labeled as "Humour", didn't you?
So the fact that the uncertainty in the measurements has gone done from about 5 billion years to 0.1 billion years means nothing to you...?
Err, no - they are about 1 billion years younger, as far as I know. And that's perfectly consistent with our ideas of stellar development.
That fits in nicely with WMAP's conclusion after looking at the cosmic background radiation and measuring the Hubble Constant, among other things. WMAP estimates the universe at 13.73 billion years old, give or take 1%.
How's that for a tie in to your other post on covers?
@12: the really neat thing about the WMAP age (which is also the age you get if you combine supernova and cluster measurements, without the CMB -- in other words, multiple lines of cosmological evidence give consistent results) is that it *is* consistent with the star data.
Back when I was in grad school in the 1990's, there was a cosmological age crisis. All indications were that the Hubble Constant was probably around 70 km/s/Mpc, although it was still very uncertain with lower values having their proponents. Meanwhile, there were theoretical reasons to suspect that the Universe was spatially flat... and except for a few visionaries, nobody was really taking the cosmological constant seriously. Put all of that together, and you get a Universe that had to be less than 10 billion years old. Meanwhile, the oldest stars were pushing 14 billion years old, which was obviously a problem!
Two things happened. Small tune-ups in the star estimations brought their ages down a wee bit. (The overall theory was absolutely fine, but some calibration numbers changed a bit with Hipparcos.) That brought the star "upper limit" closer to 13 billion years. Second, we discovered that the Unvierse is accelerating. An accelerating Universe with a Hubble constant of 72 km/s/Mpc can easily give you an age of the Universe around 14 billion years. No more cosmological age crisis!
@9:
Wouldn't any 6Li or 7Li produced be rapidly consumed by further reactions? I'd expect
6Li + 1H -> 4He + 3He
7Li + 1H -> 4He + 4He
to both be strongly favoured given the low binding energy of Li relative to He (Li is the only light nucleus that can release energy by fission). A little reading suggests that the above reactions should proceed at lower temperatures than hydrogen fusion itself, and that the presence of lithium in small stellar objects is used as a test to distinguish true hydrogen-burning stars from sub-stellar brown dwarfs.
Bjoern,
Yes, but there's a lot of truth in humor. As far as I can tell, the data points are correct.
"a metal-poor star in our Milky Way called HE 1523 is 13.2 billion years old-just slightly younger than 13.7 billion year age of the universe." http://www.usatoday.com/tech/science/space/2007-05-11-ancient-star_N.htm So there's that..
Then as you say, "So the fact that the uncertainty in the measurements has gone done from about 5 billion years to 0.1 billion years means nothing to you...?" This means that this science is very confident.
Now the really nice thing about such a confident and precise estimate is that the Big Bang theorists have put a stake in the ground (13.7 + or - 0.1 Billion years); this is as old as the universe gets. Wow!! I like that kind of assertive prediction.
This suggests a very specific family of predictions that scientists won't find an old star or cosmic process that forces a revision of the age of the universe. This predicts that the exponential increase ended in 2010; and henceforth we shall see a plateau in the measured age of the universe. Wow!! And if that's the case then the chart that I referenced will no longer be humor. The age of the universe plateau chart will be EXHIBIT A, pointed to with pride decade after decade, century after century.
OK then,
Bjoern and Rob Knob make very good sense.
I see nothing to argue with;
but let's give experiment, observations and theory some more time and watch the Age of the Universe Chart for a few more decades. Will it become EXHIBIT A or remain as humor?
@Thomas:
They are correct, yes - but they were cherry-picked so that it looks like an exponential increase! If one includes other data points, then the exponential increase vanishes. That was essentially the point of the joke!
So you say the given error margins are wrong? That they should be (much) larger? (obviously the given error bars assume that the underlying model is essentially correct, as always in physics - but equally obviously, it is not possible to include the possibility that the model is totally wrong in the error bars!)
Yes, indeed - these are quite strong predictions.
Well, the value has survived for about 10 years already; every new measurement that came in in that time (and there were quite a lot!) supported that value. How long do you want to wait? Another 10 years? 20? 50?
Bjoern
Cherry picked, I did not know that. 10 years already, I did not know that. But I believe you.
Nevertheless, I choose to wait. I allow myself to continue to be skeptical; despite the impressive logic of WMAP. 10 years? 20? 50? is not so long to wait and see what good science can discover. I'll spare you my laundry list of open questions.
In my mind it is still premature to decide upon the "age of the universe" and it is still premature to decide upon even the concept "age of the universe." Is the concept "age of the universe" any better defined than "age of a black hole" or "age of an elementary particle." I think the motive to define "age of the universe" is philosophic.
Of course, if I were to read the phrase, "apparent age of the universe is 13.7 billions years"; I would agree.
@Thomas:
Well, that's my impression, since I remember several age determinations which don't appear on that site.
10 years was a rough estimate; looking the papers up, it actually was in 2003, hence only 7 years.
Well, I told you above what the phrase means.
Bjoern
Thanks as always.
Look, the evidence is really overwhelming. I just found a quote (which I couldn't easily copy) which basically said that for the last 40 years cosmologist have said the number is between 10 and 20 billion years and then explained how astounding that is. (and it is).
So the estimate of 13.7 + or - 0.1 billion years is sweet. Frankly, without your discussion, I wouldn't have realized how strong this WMAP result is.
Yet here I am still skeptical. But hopefully neither offensive nor antiscientific in my skepticism.
On rereading this still strikes me as one of Ethan's most profound posts.
I wish I had a better grasp of stellar spectrometry. So much of what is known seems to depend on color: spectral class, distance, rate of recession, composition. And yet the starlight seems so small and far away, and subject to extinction by interstellar gas and dust, not to speak of earth's own atmosphere. How can scientists tell so much from so little? And, once they have it, how do they distinguish among the factors that contribute to a star's color profile?
It's not the colour that counts, it's the pattern of absorption lines.
The absorption lines reveal the temperature of the star's surface (and hence the colour) because they change according to the degree of ionization of the atoms present, and the presence of simple chemical compounds, which depend on the temperature as well as the composition. Spectral classes were originally defined before this was fully understood, which is why they're not in alphabetic sequence; since many of the important spectral lines correspond to high ionization levels, they were not initially identified as such, and many were initially assumed to be new elements (after the example of helium which was discovered this way).
Interstellar gas and dust may affect the overall colour of the light, but it doesn't disturb the absorption lines. Likewise, the position of those lines can be accurately measured, and (by calculating the Doppler shift) the rate of recession can be determined.
(The conventional "colour" descriptions of spectral classes have little to do with the reality anyway. Class G is labelled "yellow" (or sometimes "yellow-white"), but the Sun (spectral class G2) produces light which is white more or less by definition.)
I'm going to have to side with Thomas on this one Bjoern. Imagine how many individual electrons it would take to fill up the entire observable universe. Its been said that it would take the same number of observable universes to fill the volume of the 'net total universe.' Only difference is I'm not expecting to hear a new age theory on the universe at least in my lifetime. One can hope at least.
Thanks Andrew. That was clarifying.
If you have time, could you explain "interstellar gas and dust"? If stars are formed from gas and dust, presumably the gas is largely hydrogen, because that's what stars are mostly made of, even second-generation stars like the sun. Yet where does all the hydrogen come from, if stars burn out when they use theirs up? Is hydrogen a sort of ur-element, present from the beginning? And just what is this "dust"? Was there ur-dust as well? Or is all the dust literally stardust, the detritus of massive, live-hard-die-young, first-generation stars?
In a word, yes. The Big Bang left hydrogen as the most common element present by a large margin (about 75% by mass, with the remaining 25% being helium; small traces of lithium were formed at that time too). Only a small fraction of that initial stock of hydrogen has been consumed by stars.
The term "dust" implies particles of at least a few molecules in size; hydrogen and helium do not condense in vacuum and therefore dust can only occur as the result of reactions of heavier elements. Other than the more or less insignificant trace of lithium left by the Big Bang, all of those heavier elements were ultimately produced in stars. So no, there was no ur-dust.
Literal "stardust" is that component of interstellar dust that consists of particles condensed directly out of the hot envelopes of stars (for example in supernova explosions as the ejected material cools, or in the envelopes of red giants ejected as planetary nebulae). These tiny particles are typically diamond, silicon carbide, graphite, aluminium oxide - materials which can form hard solid grains even at high temperature. These particles are recovered intact from meteorites and larger dust grains and studied in order to gain information about stellar processes.
In other cases, material may be ejected from stars in gaseous form and condense out into solids (or form molecules, including a remarkably wide range of simple "organic", i.e. carbon-based, compounds) later as a result of collecting in regions which are sufficiently cool and dense to allow enough interaction between atoms. In some cases the dust may be vaporized again by subsequent heating or photodissociation, especially during solar system formation; this means that besides the intact stardust grains, most of the dust we find locally in the solar system has been re-condensed, possibly multiple times.
It's one of the more awe-inspiring features of modern cosmological knowledge that we now know that 90% of the human body mass is made up of elements formed in stars that died (often violently) billions of years ago. (The other 10% by mass is hydrogen.)
Thanks Andrew. It is awesome how much is known about the starry heavens above us. I'm afraid we've made rather less progress on the moral law within.
How do you know that the oldest things in the universe are not just an indication of the last time the universe completely recycled itself? Every 12 billion years or so all or most of the oldest existing marterial has been recycled into new & younger stars. These in turn live and then die, adding their material into space to be recycled again, over, and over, and over. Is that not what we are seeing happening? Old material recycling into new stars? How many times has the material in the Earth been recycled? How many stars have our atoms been a part of? If the universe is only 12 billion years old and the average star is 10 billion years old, then our 6.54 billion year old sun can only be a first generation sun crated from virgin hydrogen. If that is so then where did all our heaver elements come from?
I propose the fragility of facts and the possibility that science as we know it has stayed from truth.
I believe that our existence as part of the universe has lead to problems of perspective. Perhaps what we are calling force is in fact not but the symptom of a state of accelerating expansion. Perhaps all matter is acting like dense NRG and it is accelerating away from centers of volume at a rate that is directly in proportion to the density of the NRG in those volumes. Perhaps this acceleration has been taking place for a very long time.
If I am correct then the surface of the earth might be accelerating away from the center of the earth at â9.8 m/s². Given radiometric dating, one suggested age for the Earth is at least one kÄlpa or â4 billion years. 4 à 10â9 years à 31,556,926s/y... well you get the idea. Even though my madness would result in relative rate of expansion greater by magnitudes to that of the accepted maximum for light I still chose my madness over the idea of a force of gravity.
Dr. Albert Einstein inspires the special view of our universe by seeing from a perspective outside our experience. That point of view allows us to see our existence in a way humans have not yet done before as far as I know. "The observed independence of the speed of light on the observerâs state of motion required fundamental changes to the notion of simultaneity." What if this need for observed independence should also extend to another dimension so that an observer might imagine another aspect of reality and remain free from perceptual bias?
How can you measure without a yardstick? You cannot tell how old the universe is based on stars and the brightness of them because their distances effect their brightness. I do not know how you arrived at the hypothesis that because the stars begin to separate their age changes. In addition, for each star cluster you measure if this could work, you have to factor in an 10%+ margin for error. This really isn't a good way of measuring the universe in any way.
it is reasonable to argue that both looking inward to that smallest part of a single atom and outward to the limits of our visible universe that there is more mystery than we could ever imagine and that we are always to be the spellbound children of our tiny part of the sky till the last rays ebb from our mother sun and we like many star races are no more.
Il existe selon la Theorie-du-Tout Mecanique une autre methode pour determiner l'age de l'univers. Elle est fondee sur l'equation-du-tout, qui fait intervenir les concepts esprit-matiere et des theoremes de la Theorie mathematique LA CYCLOLOGIE. Elle demontre que l'age de l'univers ne peut etre inferieur a 13.6 milliards d'annees.
Hi,
check this video,
http://www.youtube.com/watch?v=XpFVsqTvhDU
about the satellite
this is stupid