"Build a man a fire, and he'll be warm for a day. Set a man on fire, and he'll be warm for the rest of his life." -Terry Pratchett
Whether it comes from without or within, there are few things in this life that captivate our attention better than a brilliant, hot and colorful display. This weekend, have a listen to The Roots with John Legend, as they sing about
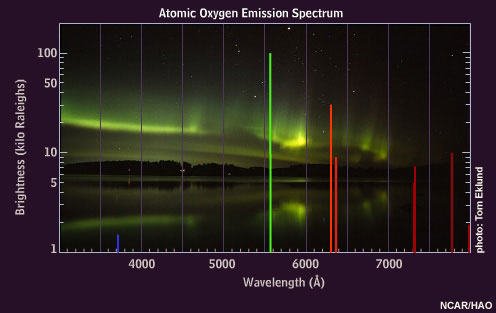
Deep off in the depths of space, although you won't find any stars burning a bright green color, you will find stellar remnants, or star corpses, glowing a brilliant green.
This has everything to do with chemistry, and particularly with the element oxygen. When you take oxygen and super-heat it -- to hot enough temperatures that you knock an electron off of the atom itself -- you create a charged ion. From that state, it eventually finds another electron to recombine with. And when this recombination happens, it emits photons of a particular frequency, which just happen to be picked up as green by our eyes.
This phenomenon, which causes many planetary nebulae to glow an eerie green, is the same one that's responsible for the brilliant aurorae, or polar lights, that occasionally light up the high-latitude skies at night.
When high-energy particles from the Sun collide with the atmosphere, they often ionize many of the molecules up there, including -- of course -- oxygen! Skywatchers in the right parts of the northern United States got an early St. Patrick's Day auroral surprise before sunrise this morning, as Bob King documented in words and pictures!
But for those of us who aren't fortunate enough to see the cosmic green fire in the heavens above, here's a little trick you can do yourself with some common household items to produce a green flame in your own backyard!
It isn't oxygen that creates this green color; it's due to the chemical reactions of boron! All you'll need is a clear-burning alcohol product -- methyl alcohol (like HEET) works better than ethyl or isopropyl alcohol (which burns orange-to-blue at various times) -- and some pure boric acid.
Mix about a half-cup to a cup of the alcohol with a teaspoon or two of the boric acid, and put them in a container than can handle a fairly intense alcohol flame: a sturdy glass jar, a metal can, or a stoneware container all work fine. Finally, outdoors for safety, go ahead and light it.
A half-cup's worth will burn for about 10 minutes in a tin can. And that's all it takes to produce a green fire; have a little St. Patrick's Day fun with this, or save it for a frightfully eerie Halloween!
Have a great weekend, all of you, no matter how you're spending it. And for those of you who prefer to look at the green lights of the aurora, you can check for them again tonight, or enjoy this lovely show from Norway, courtesy of Dr. Claus & Anneliese Possberg!







very cool.
Topical and crazy,
http://www.monkeyview.net/id/545/fire/7
Blew the link,
http://www.monkeyview.net/id/545/fire/