tags: evolution, evolutionary biology, gynandromorph, bilateral gynandromorph bird, half-sider, mixed-sex chimaera, sex determination, molecular biology, genetics, developmental biology, endocrinology, birds, chicken, Gallus gallus, ornithology, researchblogging.org,peer-reviewed research, peer-reviewed paper, journal club
Half-sider.
Almost exactly one year ago, hundreds of American birders
were thrilled by sightings and photographs of this remarkable
Northern Cardinal, or Redbird, Cardinalis cardinalis,
photographed in Warrenton, VA.
Image: DW Maiden, 2 March 2009.
I'll never forget the first time I saw a bilateral gynandromorph. I was a bird-crazy teenager reading my way through a stack of avicultural publications when I spied the strangest bird I'd ever seen on the cover of one magazine: an eclectus parrot that was very precisely divided down the middle: one side was rich scarlet and the other was brilliant emerald. Because eclectus parrots are sexually dimorphic -- females are red and males are green -- this remarkable bird was easily identifiable as being composed of both sexes, one on each side.
Even though this was the first time I'd ever seen a gynandromorph, these mysterious birds do pop up from time to time. For example, bird watchers occasionally run across them in the wild (see above photograph) and poultry farmers sometimes find them in their flocks: it is estimated that roughly one in 10,000 domestic chickens -- another sexually dimorphic species -- is a gynandromorph.
Bilateral gynandromorphs are mysterious because nothing like them has been seen in mammals, so they generate a lot of speculation whenever they turn up.
It was widely assumed that sexual development in birds and mammals follows basically the same trajectory. In almost all mammals, including humans, embryonic cells start off being "unisex"; indistinguishable regardless of which sex chromosomes they contain. Early in development, the sex-determining region of the Y chromosome (Sry) controls whether a testis or ovary forms: if Sry is present, testes grow, while ovaries develop in the absence of Sry. By the seventh week of embryonic life, the developing gonads begin secreting chemical messengers -- hormones -- that direct other cells to develop as either male or female.
Given this paradigm, scientists studying gynandromorphs were surprised to discover that sexual development in birds is dramatically different from most mammals: unlike mammals, individual chicken cells apparently "know" which sex they are at the time of fertilization and they maintain their own male or female identities throughout life.
"We assumed that sex determination in birds would follow the mammal pattern," reports Michael Clinton, a developmental biologist at the University of Edinburgh in Scotland. Because Dr Clinton has spent much of his scientific life scratching his head over why it was so difficult to find the avian Sry in chicken chromosomes, he was intrigued by these peculiar chickens and quickly assembled a team of scientists to study the birds.
Dr Clinton and his colleagues originally hypothesized that one side of bilaterally gynandromorphic birds would be a genetically normal female (or male) while the other side suffered some kind of chromosomal anomaly or mutation. In their journey to understand the molecular and cellular mechanisms that lead to a gynandromorph, these researchers made a fundamental and bizarre discovery about sexual development.
This story begins serendipitously enough. A few years ago, an employee in the poultry industry described to Dr Clinton some peculiar chickens on nearby farms. These rare chickens were bilateral gynandromorphs; half male and half female. Like my eclectus, these birds were neatly divided down the middle between their male and female sides, almost as if two individuals of opposite sexes had been stitched together.
These "half-siders," as poultry farmers and aviculturists often refer to bilateral gynandromorphs, are rare, but have been seen in a number of avian families, ranging from finches to pigeons to parrots. Most recently, a wild gynandromorphic Northern Cardinal, Cardinalis cardinalis, was photographed on the East Coast of the United States almost exactly one year ago, re-igniting interest and speculation among online birders throughout the world (see featured image at top).
Like northern cardinals, domestic chickens are sexually dimorphic. So when Dr Clinton first saw these oddly lopsided chickens, he was immediately impressed by the birds' striking appearance: the larger male side had white feathers, spurs, large wattles and breast muscles, whereas the smaller female side showed the characteristic dark coloring, small wattles, and the lack of spurs (Figure 1):
Figure 1 | Image of gynandromorph bird (G1). ISA brown bird where the right side has female characteristics and left side has male characteristics (white colour and larger wattle, breast musculature and spur).
DOI: 10.1038/nature08852
Dr Clinton's team eventually obtained three of the unusual birds, which are referred to in their newly published paper as G1, G2 and G3. All three were ISA brown birds, a commercial breed with sex-linked plumage color. ISA brown males are heterozygous for the dominant silver and recessive gold genes (Ss) so they have white plumage; females possess only the gold gene (s-) and thus have brown plumage.
Careful observations of the behaviors displayed by one of the team's gynandromorphs, G1, which came to them already named "Sam" -- Samantha for the right side and Samuel for the left -- showed that it not only looked strange, but it was also a bit confused.
Although gynandromorphs are nearly always sterile, "Sam seemed to think it was male," reports Dr Clinton. "But when we put it in with a couple of females I don't think they were too sure."
This confusion was also apparent when the birds' gonads were examined: G1 contained a testis-like gonad on the left side, G2 contained an ovary-like gonad on the left side, and G3 contained a swollen testis-like structure on the left side (in contrast to G1 and G2, G3 appeared female on the left side and male on the right).
G1's testis-like gonad was composed primarily of sperm containing seminiferous tubules, whereas the G2 ovary-like gonad was composed predominantly of large and small follicles. The gonad from G3 comprised a mixture of empty tubules and small follicularlike structures (ovo-testis).
While the researchers were observing their three odd chickens, they were also collecting tissue samples and examining the chromosomes to decipher the molecular mechanisms underlying bilateral gynandromorphs.
The team tested whether the chickens suffered from a genetic anomaly by examining the chromosomes found in tiny samples of blood as well as skin, breast muscle and wattles collected from both sides of each gynandromorph. They color-coded the chromosomes in these cells with fluorescent dyes that stick to either the Z or W sex chromosomes and examined them with confocal microscopy.
Contrary to their prediction, they found that fluorescently-labeled cells collected from opposite sides of the same bird were either predominantly normal male or female cells, while the blood was a mixture of normal male and female cells. The gynandromorphs were in fact nearly perfect male:female chimæras comprised of normal female cells with ZW sex chromosomes on one side, whereas the cockerel side contained mostly normal male cells with male sex chromosomes, ZZ (Figure 2):
Figure 2 | Male and female cells in gynandromorph birds. a, FISH analysis of sex chromosomes in gynandromorph blood cells. Shown are interphase nuclei prepared from cultured blood cells from gynandromorph G1 hybridized according to standard FISH procedures with probes specific to both the W and Z chromosome (XhoI repeat on W chromosome, and Z chromosome bacterial artificial chromosome (BAC) containing the VLDL receptor gene identified by screening the HGMP chicken BAC library). Erythrocytes were hybridized with probes for Z chromosome (green) and W chromosome (red). Cells contain either two Z chromosomes or one Z and one W chromosome. b, Mean relative proportions of ZZ and ZW cells in tissues from male and female sides of gynandromorph birds. The average percentage of ZW and ZZ cells (Supplementary Table 2 [GrrlScientist comment: data not shown here]) in three tissues from the phenotypically female side and from the phenotypically male side of three gynandromorph birds is shown. Tissues from the sides that appear female contain more ZW (female) than ZZ (male) cells, whereas tissues from the sides that appear male are composed predominantly of ZZ cells. BM, breast muscle; Sk., skin; Wa., wattle. [larger view]
DOI: 10.1038/nature08852
Because mammalian cells are strongly influenced by sex hormones after embryonic gonads begin to develop, the team wondered if this was also true for birds. They investigated whether sex differences between male and female cells exist independently of gonadal hormone influences or if individual cells assume the same sexual identity as their neighbors. To test this, they compared gene expression in male and female embryos during the developmental stages before the gonads form, and discovered that even at these early stages, male and female cells already "know" their sexual identity by showing sex-specific gene expression profiles (Figure 3):
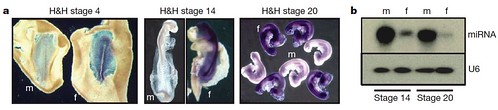
Figure 3 | Sexually dimorphic expression in early chick embryos. a, Expression of FAF in male and female embryos before development of genital ridge/gonads. Whole-mount ISH showing expression of FAF (purple) in embryos at 18 h, 48 h and 72 h of development (H&H stages 4 (original magnification, 340), 14 (320) and 20 (310), respectively). FAF is clearly expressed throughout the female embryos at all developmental stages and is not expressed in male embryos. FAF is not expressed in extraembryonic tissues of the female. The FAF transcript is encoded by the genomic DNA complementary to the intergenic regions between copies of the W-chromosome repeat gene Wpkci (also called HINTW) and transcribed in the opposite orientation. f, female; m, male. b, Expression of novel chicken miRNA (Gallus gallus mir-2954). Expression in whole embryos at 48 h (H&H 14) and 72 h (H&H 20) of development is shown. This miRNA is clearly expressed in a sexually dimorphic fashion at stages before the sexual differentiation of the gonads. This miRNA matches sequence present in chicken Z-chromosome BAC clones AC192757 and AC187119. U6 RNA was used as a loading control. [larger view]
DOI: 10.1038/nature08852
Intrigued, the team then tested whether individual cells respond to the overwhelmingly male or female environment seen on each side of bilateral gynandromorphs -- do male cells "conform" to a mostly female environment by assuming a female role, and vice versa? To examine this possibility, they created embryos containing chimæric gonads by embedding female cells in male host tissue and vice versa. They found that male donor cells embedded in female host cells didn't take on female roles. Similarly, female donor cells embedded in male host cells didn't assume male functions, either (Figure 4):
Figure 4 | Expression of male and female markers in chimæric gonads. a, Generation of chimæras. Left: schematic illustrating transplantation of presumptive mesoderm from GFP-expressing embryo to non-GFP embryo at day 2. Right: image of mesonephros and gonads from chimæric embryo at day 9 showing donor contribution to left gonad (g), mesonephros (m) and Müllerian duct (md). Original magnification, 320. b, Expression of female and male markers in embryonic gonads. Expression of aromatase (AROM) in ovary and anti-Müllerian hormone (AMH) in testis at day 9 is shown by IHC. Original magnification, 3400. c, Integration of GFP-expressing donor cells into host gonads. Panels in the first column show a low-magnification view of sections through host gonads and illustrate the extent of donor cell contribution. Panels to the right show higher-magnification views of highlighted areas. Using IHC, donor cells are marked by GFP (green) whereas expression of AMH and aromatase are shown in red. The fourth column is a merged image of the images from the second and third columns. In same-sex chimæras, GFP-expressing donor cells co-localize with AMH expressing and aromatase-expressing cells in host testis and ovary, respectively (yellow/orange in the fourth column). In mixed-sex chimæras, GFP-expressing donor cells do not co-localize with AMH or aromatase. m, mesonephros; o, ovary; t, testis. d, Retention of female donor phenotype in mixed-sex chimæras. IHC showing expression of AMH(red in top row) and aromatase (red in bottom row) in neighbouring sections from the gonad of a female:male (donor:host) chimæra. Donor contribution is illustrated by GFP (green) expression. The right column shows a merged image of the images in left and middle columns. Regions containing a significant host contribution (defined by the bottom bracket) formed sex-cord-like structures and expressed AMH. Female donor cells were not incorporated into AMH-expressing sex cords, as shown by the lack of GFP and AMH colocalization. Regions composed primarily of female donor cells (defined by top bracket) behaved as ovarian-like tissue and expressed aromatase, as shown by co-localization of GFP and aromatase (yellow/orange). Scale bars in c and d indicate 100 μm. IHC was performed following standard procedures. Primary antibodies were (1:100) goat anti-human AMH (Santa Cruz Biotechnology), (1:200) mouse anti-human cytochrome P450 aromatase (AbD Serotec) and (1:250) rabbit anti-GFP conjugated to Alexa Fluor 488 (Invitrogen). Secondary antibodies were conjugated to Alexa Fluor 594 (Invitrogen). [larger view]
DOI: 10.1038/nature08852
Based on these data, the team concluded that individual avian cells maintain their own sexual identity and were not able to switch sexual roles despite developmental signals from nearby cells: their gender orientation was fixed from their inception.
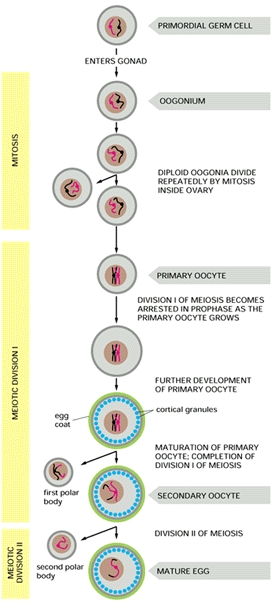
Since these data clearly establish the presence of both ZZ- and ZW-containing cells, the team realized that it is highly unlikely that gynandromorphs arise as a consequence of either a mutation or a loss of a sex chromosome at the two-cell stage of development, as they originally predicted. Thus the team proposed that bilateral gynandromorphs start at the very beginning: they result from the failure of the developing ovum to extrude a polar body during meiosis (refer to figure at right). When this abnormal ovum containing two pronuclei is fertilized (by two sperm, a situation known as polyspermy), it contains both a Z- and W-containing nucleus, which then give rise to each half of the whole bird -- a bilateral gynandromorph. (Avian sperm only contain the Z sex chromosome, whilst a mature egg cell has either a Z or W sex chromosome.)
Further, because both sides of the bird are exposed to exactly the same hormones, individual cells respond to these chemical signals according to their own inherent chromosomal complement rather than simply following orders sent out by the gonads.
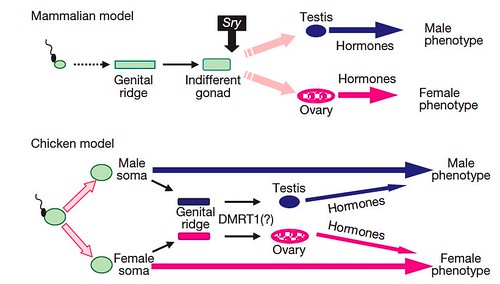
Based on their studies, the team proposed two different models of sexual development; one for birds and a second, contrasting model to account for most mammals (Figure 5):
Figure 5 | A novel mechanism of sex determination in the chicken. A sexual identity is genetically imposed on the male and female chicken soma at fertilization and is the major factor in determining the adult sexual phenotype. At the appropriate stage in development, the sexually-dimorphic transcripts underlying the male/female identity trigger expression in the genital ridge of the gene cascade responsible for testis/ovary development. The gonads have limited effects on the sexual phenotype. In contrast, in mammals, gonadal fate is dependent on transient expression of the testis-determining Sry gene in the indifferent early gonad. The mammalian gonads have a major influence on the sexual phenotype. [larger view]
DOI: 10.1038/nature08852
Interestingly, birds aren't the only exceptions to the mammalian model of sexual development. The mammal model also fails for some marsupials. Previous studies have shown that formation of mammary glands and scrotum in the wallaby, a marsupial, is independent of gonadal hormones [DOI: 10.1038/331716a0]. Additionally, the platypus (a monotreme) has a complex sex chromosome system that is unique among mammals [DOI: 10.1038/nature06936] documented so far, although like birds, they also lack the mammalian sex determining region (Sry). It would be interesting to identify whether there are platypus gynandromorphs and if so, to find out if each cell in the platypus has its own sex identity, as in birds.
Interestingly, sex determination in reptiles appears closer to the mammalian system but again, it's complicated, and there are species where sex is dependent upon ambient temperature experienced during specific stages of embryonic development.
Of course, this makes me wonder if any bilateral gynandormorphic Maniraptoran fossils have ever been identified?
"The problem is, once people develop a hard and fast rule, it becomes the only game in town," explained Dr Clinton. Certainly, Sam's "tubes and plumbing" would suggest there is no universal rule for all vertebrates.
Source:
Zhao, D., McBride, D., Nandi, S., McQueen, H., McGrew, M., Hocking, P., Lewis, P., Sang, H., & Clinton, M. (2010). Somatic sex identity is cell autonomous in the chicken. Nature, 464 (7286), 237-242 DOI: 10.1038/nature08852






Huh. I thought it was lots more complicated than that.
(my emphasis)
Ref:
====
Warren, W. C. et al. 2008. Genome analysis of the platypus reveals unique signatures of evolution. Nature 453, 175-184. doi: 10.1038/nature06936
Refs in paper:
==============
16. El-Mogharbel, N. et al. DMRT gene cluster analysis in the platypus: new insights into genomic organization and regulatory regions. Genomics 89, 10â21 (2007).
17. Rens, W. et al. Resolution and evolution of the duck-billed platypus karyotype with an X1Y1X2Y2X3Y3X4Y4X5Y5 male sex chromosome constitution. Proc. Natl Acad. Sci. USA 101, 16257â16261 (2004).
18. Grutzner, F. et al. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432, 913â917 (2004).
On the other manus....
F. Veyrunes et. al. Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes Genome Res. 2008. 18: 965-973 doi: 10.1101/gr.7101908
Are they hypothesizing that the egg fails to extrude the second polar body and both are fertilized, requiring two sperm? Or are they hypothesizing that the egg fails to extrude the first polar body, which then develops parthenogenetically into a 2N individual? Is that even possible for a 2N cell whose duplicate chromosomes are attached to one another at the centromeres?
Also, are either of these possible explanations for the apparently gynandromorphic baby reported in Karam, J.A. and L. A. Baker, 2004. True Hermaphroditism. NEJM 350 (4): 393 ?
Thank you for your detailed explanation and pictures. I was very curious about this after hearing it on the radio. Yours has been the easiest to understand detailed explanation I've found.
So, Owlmirror, not only are platypuses designed by committee, their sex is too.
Hm. I see that the text changed slightly from what I originally blockquoted.
I think the sentence would be less confusing all around if you simply removed the "ZW" term, there -- that retains the same sense without giving the mistaken impression that platypus use the same sex-determination system as birds, yet avoids going into the details of the confusing and complex system that they do use.
owlmirror: okay, i incorporated your suggestion into the story text (still poking at this story -- yes, it's a rough draft! -- so the text will likely change more during the next 24 hours, especially after/if the authors answer my emailed questions).
i would have mentioned the change in the original in this comment thread, but it was 2am when i was finally done for the night, and i was simply too exhausted to do anything except crawl into bed.
Excellent post! This is fascinating material, and you presented it very approachably for readers less specialized in developmental biology.
This post was selected in "Surveying the gut microbiota, cross dressing chickens and more, in my Picks of the Week, from RB"
http://amontenegro.blogspot.com/2010/03/surveying-gut-microbiota-cross-…