Earlier this week, I wrote about how the heavier elements in the Universe were made. Specifically, that they are made in stars. These stars then explode in a variety of ways, enriching the Universe with these heavy elements, and allowing us to form glorious things, like our planet!

By contrast, the big bang makes light elements, but not heavy ones. Why is this the case, and how do we know? Let's find out.
Just a few seconds after the big bang, the Universe is filled with protons and neutrons, in roughly the same numbers as we have today. But, none of these protons and neutrons are bound together, they're all free and lonesome. Why? There's certainly enough energy for them to interact with one another, and there's certainly a high enough density for them to interact frequently. But -- in addition to protons and neutrons -- the Universe is full of photons, or particles of pure energy. These are important because photons outnumber protons-and-neutrons by almost 2,000,000,000 to 1! So even though a proton and neutron can fuse together to make deuterium at any time:
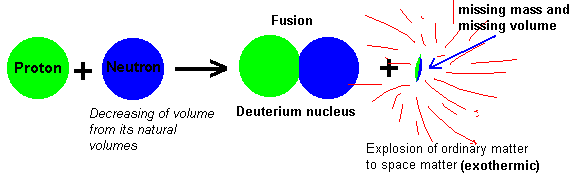
the photons are so energetic and so abundant that they immediately blast the deuterium back into a proton and neutron. This continues until the Universe has cooled enough for deuterium to be stable, which doesn't happen until the Universe is a little more than three minutes old. Once the Universe cools enough, protons and neutrons can combine to not only form deuterium, but also tritium, Helium-3, and Helium-4. And it does this pretty easily, so that relatively quickly over 99.98% of the Universe is either a Helium-4 nucleus (2 protons and 2 neutrons) or a Hydrogen nucleus (1 proton alone).
But now, we've got a problem. We can't really go up from Helium-4. By this point, a little more than 3 minutes after the Big Bang, the Universe is already 1,000,000,000 times less dense than the interior of the Sun. When densities are low, it means that reactions occur at lower rates. So let's take our Helium-4 and try adding things to it:
- Helium-4 + Hydrogen = Lithium-5, which is completely unstable and decays back into a proton and a helium nucleus after less than 10^-21 seconds.
- Helium-4 + deuterium = Lithium-6, which is stable, and of which trace amounts (about 1 nucleus in 10,000,000,000) still exist.
- Helium-4 + tritium = Lithium-7, which is stable, and again, trace amounts of it (again, about 1 in 10,000,000,000 nuclei) still exist.
- Helium-4 + Helium-3 = Beryllium-7, which lives about 53 days, and then decays into Lithium-7, which we observe.
- Helium-4 + Helium-4 = Beryllium-8, which decays back into two helium nuclei after about 10^-16 seconds.
This is a huge problem, because most of the Universe (at this point) is Helium-4 and Hydrogen! But because there are no stable mass 5 or mass 8 nuclei, the Universe gets stuck. It isn't until millions of years later, when nuclei have become neutral atoms, trillions and trillions of atoms have collapsed into dust clouds, and the first stars begin to form, that we can start creating interesting elements.

Why? Because in the interior of stars, the density is suddenly high enough that 10^-16 seconds is long enough for a third Helium-4 nucleus to come along, and cause this to happen:
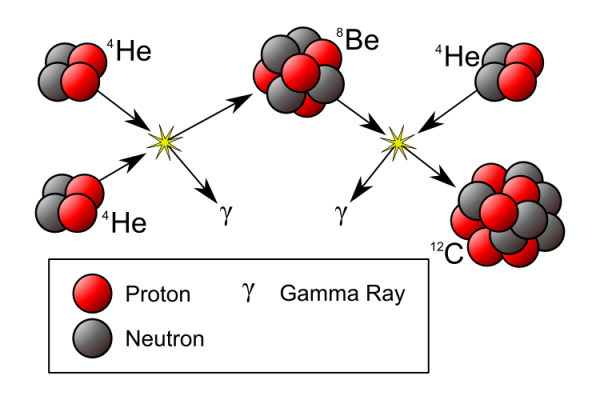
Finally! We can start forming heavier elements! It's easy from here: Carbon + Helium-4 = Oxygen, Oxygen + Helium-4 = Neon, Oxygen + Carbon = Silicon, and you not only get the picture, you get all the elements capable of making this:

When these stars with all of these heavy elements in them die, they spread their elements all throughout their galaxy; everything on planet Earth, with the possible exception of some hydrogen, was once part of a star. Not only that, but every element on Earth, again with the exception of hydrogen, was made in a star. Including every element in your body.

As Carl Sagan said, "We are all starstuff." Many of the stars you see in the sky will one day give their heavy elements up to the rest of the galaxy, and will lead to new worlds, and possibly new life. And perhaps this helps better explain why Fred Hoyle's discovery of how to make heavy elements in stars was so profoundly important.


"We are all starstuff."
Which sounds so much better than "we are all starpoop".
A great followup to the previous post.
No extra details about PPI-IV or the CNO catalytic cycle?
That was beautiful.
So I guess it's a good thing ERV does biology instead of astronomy.
Really?
I find it hard to imagine that, at a rate of expansion so fast, any gases could have later accreted enough to form a star.. I guess that just makes me ignorant.. but wow...
smijer,
It isn't too hard to figure out what the density of the Universe is at any given time. Here's how, if you're into math:
The temperature of the Universe, today, is 2.725 Kelvin. Take whatever age of the Universe you'd like to consider, and figure out what the temperature is then. For a 3-minute old Universe, the temperature is about 1 billion Kelvin.
The density of the Universe at whatever temperature you want is the density today multiplied by (Temperature then/ Temperature today)^3. If the density today is around 1 proton / cubic meter, then back then, it was about 5 x 10^25 protons / cubic meter.
The density of the Sun's interior is around 160000000 grams per cubic meter. So, multiply that by avogadro's number to get the number of protons per cubic meter in there, and you get about 10^32.
So, the early Universe, when we're making the light elements, is "only" about 2 million times less dense than the center of the Sun. Still, that's pretty significant!
That's pretty neat. I'd like to know how you found the temperature at 3 minutes? Is this derived somehow from the CMB?
I knew all this, but this article is terrific and succinct. Thanks Ethan (with help from Fred Hoyle).
I'm assuming that someone has done the accounting and the geometry and the dynamics that demonstrates that the clouds of elements expelled by the nova and supernova of the first generation stars can aggregate into gen 2 solar systems. So, who did this work and just what did the Milky Way look like back when it was all Gen 1 stars?
Hmm ... so what is the decay time of 8Be? I could swear it was 10^-17 in the previous article but only 10^-16 here.
"...get all the elements capable of making this: ..."
No you don't. There are essential elements for life that are heavier than iron, e.g. copper, selenium, and some lanthanides. I suppose you will generate them in the next part...
Is there a good book that explains all this stuff? Something about the same intellectual level as this post (very good by the way)? Back in the day when we all sat listening to Pink Floyd in the dark, half drunk, talking about the universe, quarks were all a bit strange...and some were charming, too :0) I need an update...
"No you don't" -lol Nice catch but aren't these heavier than iron elements also produced in stars? -I'm scientifically illiterate but am nonetheless fascinated.
Lassi and bunnycatch3r,
You can produce elements that are slightly heavier than iron in stars, but only in small quantities. Iron-56 is the most energetically stable atom, so there's no way to fuse something to Iron-56 unless you have a tremendous amount of extra energy.
The only place we know of where this happens is in supernovae, which is the death-knell of the largest stars. So the heavier-than-iron elements do form in stars, but only at the very, very violent end of their lives.
ââ¦in addition to protons and neutrons â the Universe is full of photonsâ¦â and ââ¦millions of years later, when nuclei have become neutral atomsâ¦â
So where did the electrons come from?
I think I read in John Gribbin's The Universe that there are traces of sugar in the cosmos. But I only understood about 3% of that entire book so I could be wrong.
Nice walk through.
"We are all starstuff." [Sagan]
"We are all star struck." [~ Warhol]
Thank you for sharing your knowledge on starstuff. I have a question about the speed of light related to Black Holes.
We know that gravity has the power to bend light.
Does light slow down and come to a stop near a black hole?
Does light enter into the black hole faster than the known speed of light?
Do you believe this may be doors to parallel universes?
Thank you!
Scott
I have not seen a single photo of an element being formed .
Why ?
I am a believer,but all I get is verbiage.
Peut on avoir ses infos mé + détaillés é - scientifik lexikement parlant
Want to know the total number of elements as of 2010.
Why does no-one list them and let us know the total number?
bob johnson
ok say u have a {-} (-) whats that make a negative right then theirs a (+) (-) thats = lets see what u can make of that.... see theire the (positive) and the )negative( in the story) (plain good guy vs bad guy( right (the good guy always wins) (good guy cancels bad guy out in the story) (but the hero) (dies in the end) thats (balance( in the universe see theirs an example of balance (good vs)( (the opposers)
On the question about black holes,and if light slows down the closer it gets to the centre. I believe that the distortion curve will give the impression that the rate of travel has slowed, but in truth its speed can only maximise to the speed that light can travel at. Where ever you are in proximity to a black hole, your perception of speed will be relative. Therefore, at the centre you`re acccellerating while the distant observer will see a gradual decellaration, coming to what will seem like limbo for the outer limit observer.
Awesome site!!! Was looking for something to visually convey the concept of unlimited and lucked into finding you.
Well done!
I LIKE THE UNIVERS
what a load of rubbish. You are trying to tell us that the protons and neutrons came AFTER the big bang. So where did the atoms come from to allow for this big bang ? Are you saying that SPACE mad them??? How? You scientists talk a load of crap. A high energy heat spot is supposed to be the cause of the big bang and I ask again, where did the atoms come from to cause this high energy ???? As everything came from the first explosion, exploding stars are not required to supply the universe with heavy elements as they are already in the system. Stars were not made in clouds as the clouds do not have enough pull to squeeze the matter into a star and as there is not any solid matter around the cloud, what keeps it in place to produce enough pressure.
"You are trying to tell us that the protons and neutrons came AFTER the big bang."
Yes. YOU are trying to find a reason to get angry about it.
"So where did the atoms come from to allow for this big bang ?"
From the photons and massive (now sub-atomic) particles that could condense in the cooling universe and combine into protons and neutrons.
You DO know that they are made up of quarks, right? They're not indivisible unto themselves.
Your incredulity is manufactured out of deliberate ignorance.
"and I ask again, where did the atoms come from to cause this high energy ???"
Nobody says it was high energy. Just dense. Like yourself, but in a different manner.
"As everything came from the first explosion"
It wasn't an explosion.
"Stars were not made in clouds as the clouds do not have enough pull to squeeze the matter into a star"
Yes they do.
"what keeps it in place to produce enough pressure."
Nothing "keeps it in". They collapse by gravitational attraction making them come to a slightly more dense area and while passing through there hit one another, which throws one further out and equally lets one stay closer in. The closer in one can do the same again. And again. Until it is near the centre and makes that slightly denser area even more dense. Which brings even more atoms in. Who then do like it did and make the area even denser.
And so it feeds back on itself until there is enough mass there to make the star dense enough at its centre to make temperatures there high enough to start fusing the elements, and the star is born.
"I have not seen a single photo of an element being formed ."
Have you seen a photo of a living dinosaur?
"Why does no-one list them and let us know the total number?"
It is. Go to wikipedia and look for "Periodic table".